Input interpretation

period 3 elements | electron affinity
Summary
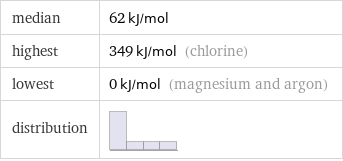
median | 62 kJ/mol highest | 349 kJ/mol (chlorine) lowest | 0 kJ/mol (magnesium and argon) distribution |
Units

Plot
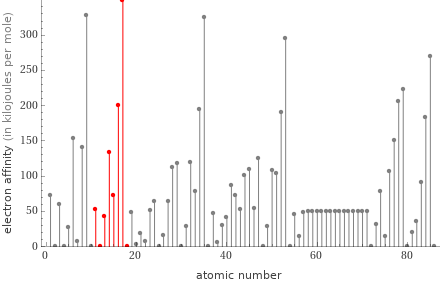
Plot
Periodic table location
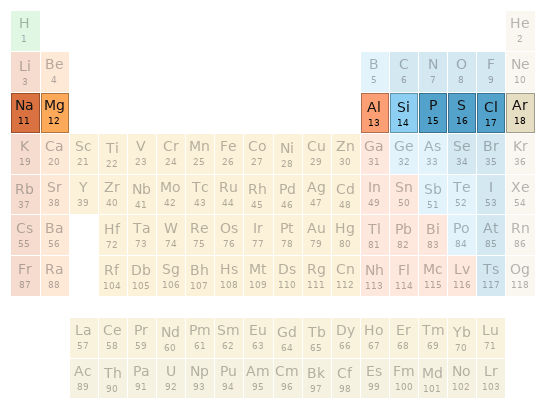
Periodic table location
Electron affinity rankings
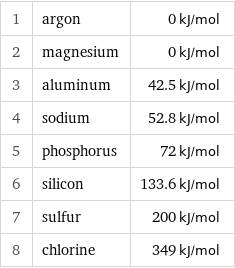
1 | argon | 0 kJ/mol 2 | magnesium | 0 kJ/mol 3 | aluminum | 42.5 kJ/mol 4 | sodium | 52.8 kJ/mol 5 | phosphorus | 72 kJ/mol 6 | silicon | 133.6 kJ/mol 7 | sulfur | 200 kJ/mol 8 | chlorine | 349 kJ/mol
Unit conversions for median electron affinity 62 kJ/mol
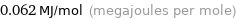
0.062 MJ/mol (megajoules per mole)
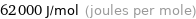
62000 J/mol (joules per mole)
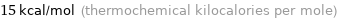
15 kcal/mol (thermochemical kilocalories per mole)
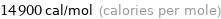
14900 cal/mol (calories per mole)
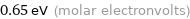
0.65 eV (molar electronvolts)