Input interpretation
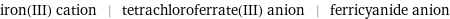
iron(III) cation | tetrachloroferrate(III) anion | ferricyanide anion
Structure diagrams

Structure diagrams
General properties
![| iron(III) cation | tetrachloroferrate(III) anion | ferricyanide anion formula | Fe^(3+) | ([FeCl_4])^- | ([Fe(CN)_6])^(3-) net ionic charge | +3 | -1 | -3 alternate names | ferric | iron(III) | iron(3+) | tetrachloroferrate(III) | tetrachloroferrate(1-) | hexacyanoferrate(III) | ferricyanide | ferricyanide(3-)](../image_source/fa434563e2ec23f97f3f94f1d645bae0.png)
| iron(III) cation | tetrachloroferrate(III) anion | ferricyanide anion formula | Fe^(3+) | ([FeCl_4])^- | ([Fe(CN)_6])^(3-) net ionic charge | +3 | -1 | -3 alternate names | ferric | iron(III) | iron(3+) | tetrachloroferrate(III) | tetrachloroferrate(1-) | hexacyanoferrate(III) | ferricyanide | ferricyanide(3-)
Ionic radii
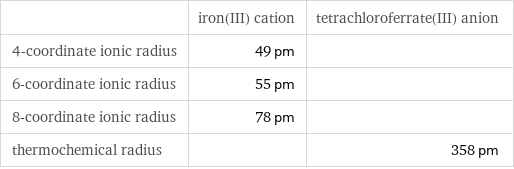
| iron(III) cation | tetrachloroferrate(III) anion 4-coordinate ionic radius | 49 pm | 6-coordinate ionic radius | 55 pm | 8-coordinate ionic radius | 78 pm | thermochemical radius | | 358 pm
Units

Other properties
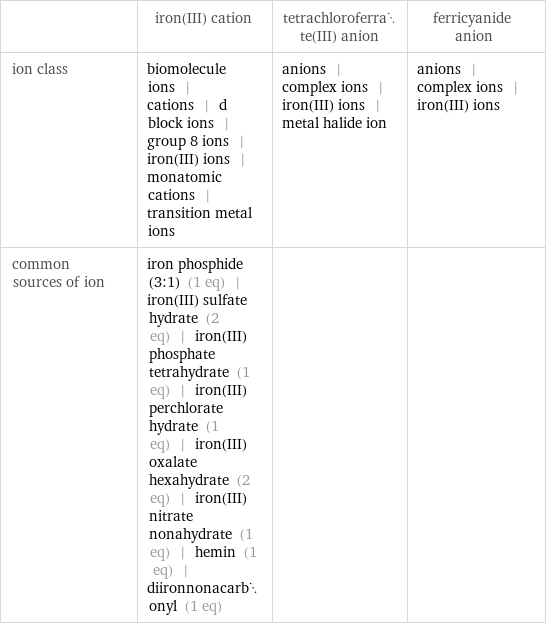
| iron(III) cation | tetrachloroferrate(III) anion | ferricyanide anion ion class | biomolecule ions | cations | d block ions | group 8 ions | iron(III) ions | monatomic cations | transition metal ions | anions | complex ions | iron(III) ions | metal halide ion | anions | complex ions | iron(III) ions common sources of ion | iron phosphide (3:1) (1 eq) | iron(III) sulfate hydrate (2 eq) | iron(III) phosphate tetrahydrate (1 eq) | iron(III) perchlorate hydrate (1 eq) | iron(III) oxalate hexahydrate (2 eq) | iron(III) nitrate nonahydrate (1 eq) | hemin (1 eq) | diironnonacarbonyl (1 eq) | |