Input interpretation
uranium hexafluoride
Chemical names and formulas

formula | F_6U name | uranium hexafluoride IUPAC name | hexafluorouranium alternate names | hexafluorouranium | uranium fluoride | uranium fluoride (1:6) | uranium hexafluoride, fissile excepted mass fractions | F (fluorine) 32.4% | U (uranium) 67.6%
Structure diagram
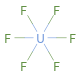
Structure diagram
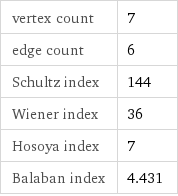
vertex count | 7 edge count | 6 Schultz index | 144 Wiener index | 36 Hosoya index | 7 Balaban index | 4.431
Basic properties
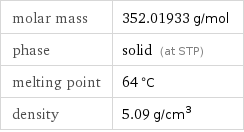
molar mass | 352.01933 g/mol phase | solid (at STP) melting point | 64 °C density | 5.09 g/cm^3
Units

Solid properties (at STP)
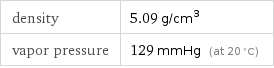
density | 5.09 g/cm^3 vapor pressure | 129 mmHg (at 20 °C)
Units

Thermodynamic properties
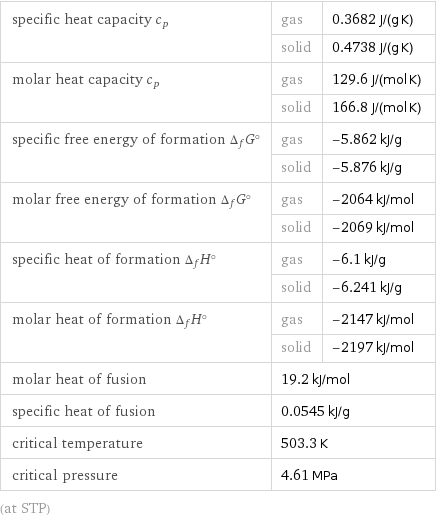
specific heat capacity c_p | gas | 0.3682 J/(g K) | solid | 0.4738 J/(g K) molar heat capacity c_p | gas | 129.6 J/(mol K) | solid | 166.8 J/(mol K) specific free energy of formation Δ_fG° | gas | -5.862 kJ/g | solid | -5.876 kJ/g molar free energy of formation Δ_fG° | gas | -2064 kJ/mol | solid | -2069 kJ/mol specific heat of formation Δ_fH° | gas | -6.1 kJ/g | solid | -6.241 kJ/g molar heat of formation Δ_fH° | gas | -2147 kJ/mol | solid | -2197 kJ/mol molar heat of fusion | 19.2 kJ/mol | specific heat of fusion | 0.0545 kJ/g | critical temperature | 503.3 K | critical pressure | 4.61 MPa | (at STP)
Chemical identifiers
(F)(F)(F)F InChI identifier | InChI=1/6FH.U/h6*1H;/q;;;;;;+6/p-6/f6F.U/h6*1h;/q6*-1;m/rF6U/c1-7(2, 3, 4, 5)6 EU number | 232-028-6 Gmelin number | 2923 RTECS number | YR4720000](../image_source/900716c76539e9f2ad6aa1c1e8f5ddcc.png)
CAS number | 7783-81-5 PubChem CID number | 24560 SMILES identifier | F[U](F)(F)(F)(F)F InChI identifier | InChI=1/6FH.U/h6*1H;/q;;;;;;+6/p-6/f6F.U/h6*1h;/q6*-1;m/rF6U/c1-7(2, 3, 4, 5)6 EU number | 232-028-6 Gmelin number | 2923 RTECS number | YR4720000
Toxicity properties

RTECS classes | other