Input interpretation

period 2 elements | specific heat at STP
Summary
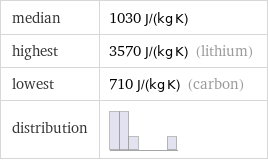
median | 1030 J/(kg K) highest | 3570 J/(kg K) (lithium) lowest | 710 J/(kg K) (carbon) distribution |
Units

Ranked values
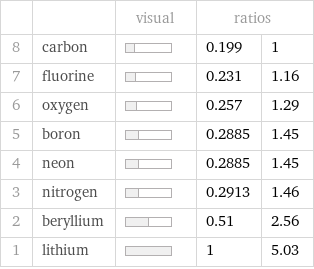
| | visual | ratios | 8 | carbon | | 0.199 | 1 7 | fluorine | | 0.231 | 1.16 6 | oxygen | | 0.257 | 1.29 5 | boron | | 0.2885 | 1.45 4 | neon | | 0.2885 | 1.45 3 | nitrogen | | 0.2913 | 1.46 2 | beryllium | | 0.51 | 2.56 1 | lithium | | 1 | 5.03
Specific heat at STP rankings
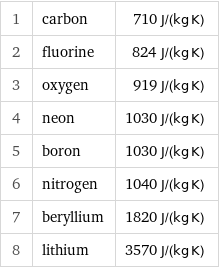
1 | carbon | 710 J/(kg K) 2 | fluorine | 824 J/(kg K) 3 | oxygen | 919 J/(kg K) 4 | neon | 1030 J/(kg K) 5 | boron | 1030 J/(kg K) 6 | nitrogen | 1040 J/(kg K) 7 | beryllium | 1820 J/(kg K) 8 | lithium | 3570 J/(kg K)
Unit conversions for median specific heat at STP 1030 J/(kg K)
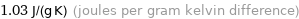
1.03 J/(g K) (joules per gram kelvin difference)
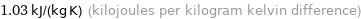
1.03 kJ/(kg K) (kilojoules per kilogram kelvin difference)
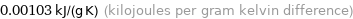
0.00103 kJ/(g K) (kilojoules per gram kelvin difference)
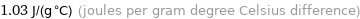
1.03 J/(g °C) (joules per gram degree Celsius difference)
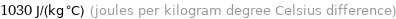
1030 J/(kg °C) (joules per kilogram degree Celsius difference)

0.246 BTU_IT/(lb °F) (IT British thermal units per pound degree Fahrenheit difference)
Comparisons for median specific heat at STP 1030 J/(kg K)
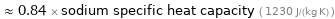
≈ 0.84 × sodium specific heat capacity ( 1230 J/(kg K) )
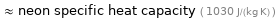
≈ neon specific heat capacity ( 1030 J/(kg K) )
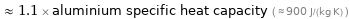
≈ 1.1 × aluminium specific heat capacity ( ≈ 900 J/(kg K) )
Thermodynamic properties
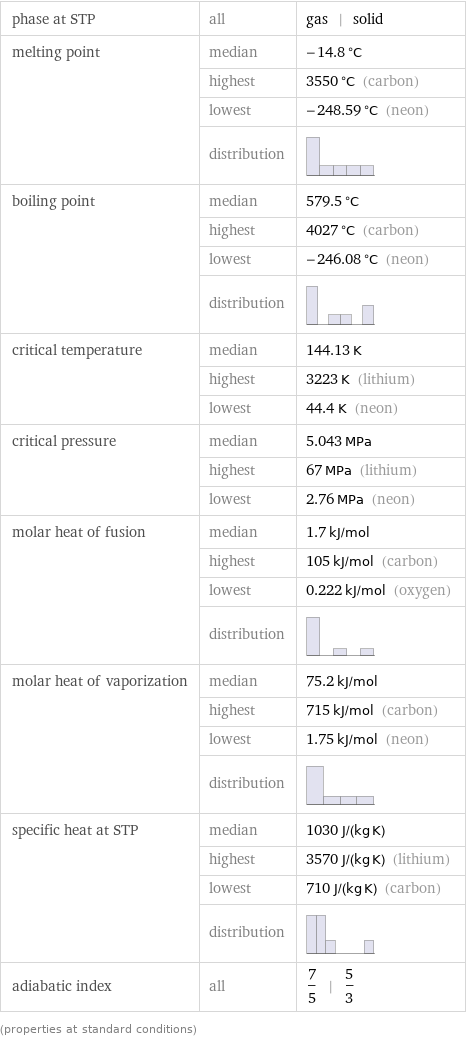
phase at STP | all | gas | solid melting point | median | -14.8 °C | highest | 3550 °C (carbon) | lowest | -248.59 °C (neon) | distribution | boiling point | median | 579.5 °C | highest | 4027 °C (carbon) | lowest | -246.08 °C (neon) | distribution | critical temperature | median | 144.13 K | highest | 3223 K (lithium) | lowest | 44.4 K (neon) critical pressure | median | 5.043 MPa | highest | 67 MPa (lithium) | lowest | 2.76 MPa (neon) molar heat of fusion | median | 1.7 kJ/mol | highest | 105 kJ/mol (carbon) | lowest | 0.222 kJ/mol (oxygen) | distribution | molar heat of vaporization | median | 75.2 kJ/mol | highest | 715 kJ/mol (carbon) | lowest | 1.75 kJ/mol (neon) | distribution | specific heat at STP | median | 1030 J/(kg K) | highest | 3570 J/(kg K) (lithium) | lowest | 710 J/(kg K) (carbon) | distribution | adiabatic index | all | 7/5 | 5/3 (properties at standard conditions)