Input interpretation
zirconium(III) chloride | ammonium nitrate-15 N
Chemical names and formulas
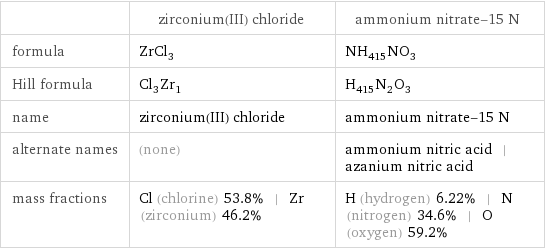
| zirconium(III) chloride | ammonium nitrate-15 N formula | ZrCl_3 | NH_415NO_3 Hill formula | Cl_3Zr_1 | H_415N_2O_3 name | zirconium(III) chloride | ammonium nitrate-15 N alternate names | (none) | ammonium nitric acid | azanium nitric acid mass fractions | Cl (chlorine) 53.8% | Zr (zirconium) 46.2% | H (hydrogen) 6.22% | N (nitrogen) 34.6% | O (oxygen) 59.2%
Structure diagrams
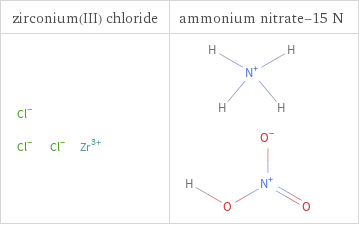
Structure diagrams
Basic properties
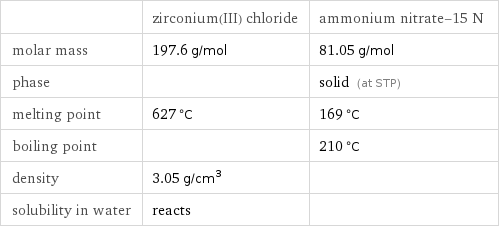
| zirconium(III) chloride | ammonium nitrate-15 N molar mass | 197.6 g/mol | 81.05 g/mol phase | | solid (at STP) melting point | 627 °C | 169 °C boiling point | | 210 °C density | 3.05 g/cm^3 | solubility in water | reacts |
Units
