Input interpretation

liquid elements (at STP) | molar heat of vaporization
Results

bromine | 14.8 kJ/mol (kilojoules per mole) mercury | 59.2 kJ/mol (kilojoules per mole)

liquid elements (at STP) | molar heat of vaporization | 37 kJ/mol (kilojoules per mole) (median)
Relative values
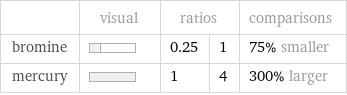
| visual | ratios | | comparisons bromine | | 0.25 | 1 | 75% smaller mercury | | 1 | 4 | 300% larger
Unit conversions for median molar heat of vaporization 37 kJ/mol
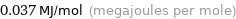
0.037 MJ/mol (megajoules per mole)
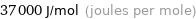
37000 J/mol (joules per mole)

8.84 kcal/mol (thermochemical kilocalories per mole)
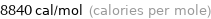
8840 cal/mol (calories per mole)

0.383 eV (molar electronvolts)