Input interpretation
dichloroaurate(I) anion | ion class
Result
anions | complex ions | gold(I) ions
Ionic radii
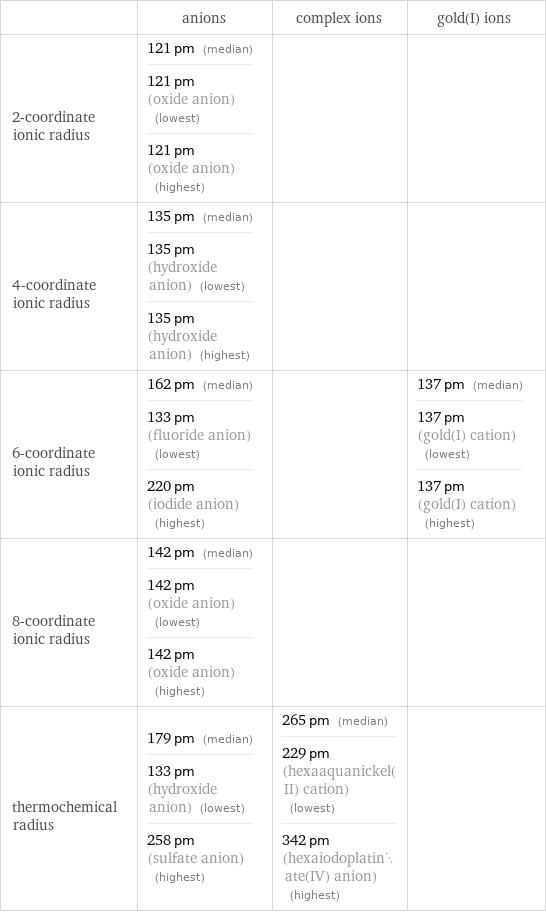
| anions | complex ions | gold(I) ions 2-coordinate ionic radius | 121 pm (median) 121 pm (oxide anion) (lowest) 121 pm (oxide anion) (highest) | | 4-coordinate ionic radius | 135 pm (median) 135 pm (hydroxide anion) (lowest) 135 pm (hydroxide anion) (highest) | | 6-coordinate ionic radius | 162 pm (median) 133 pm (fluoride anion) (lowest) 220 pm (iodide anion) (highest) | | 137 pm (median) 137 pm (gold(I) cation) (lowest) 137 pm (gold(I) cation) (highest) 8-coordinate ionic radius | 142 pm (median) 142 pm (oxide anion) (lowest) 142 pm (oxide anion) (highest) | | thermochemical radius | 179 pm (median) 133 pm (hydroxide anion) (lowest) 258 pm (sulfate anion) (highest) | 265 pm (median) 229 pm (hexaaquanickel(II) cation) (lowest) 342 pm (hexaiodoplatinate(IV) anion) (highest) |