Input interpretation
ethylene oxide | trimethylamine
Chemical names and formulas
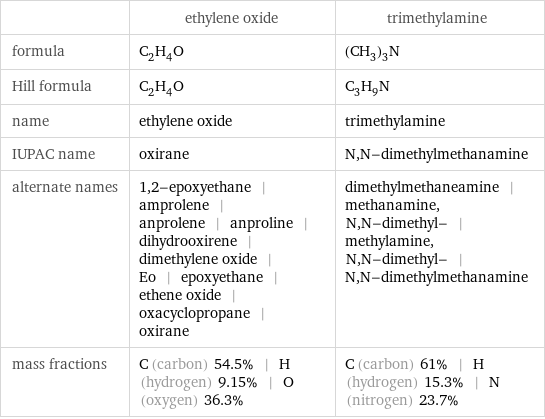
| ethylene oxide | trimethylamine formula | C_2H_4O | (CH_3)_3N Hill formula | C_2H_4O | C_3H_9N name | ethylene oxide | trimethylamine IUPAC name | oxirane | N, N-dimethylmethanamine alternate names | 1, 2-epoxyethane | amprolene | anprolene | anproline | dihydrooxirene | dimethylene oxide | Eo | epoxyethane | ethene oxide | oxacyclopropane | oxirane | dimethylmethaneamine | methanamine, N, N-dimethyl- | methylamine, N, N-dimethyl- | N, N-dimethylmethanamine mass fractions | C (carbon) 54.5% | H (hydrogen) 9.15% | O (oxygen) 36.3% | C (carbon) 61% | H (hydrogen) 15.3% | N (nitrogen) 23.7%
Structure diagrams
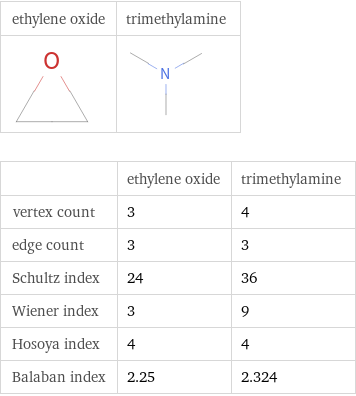
| ethylene oxide | trimethylamine vertex count | 3 | 4 edge count | 3 | 3 Schultz index | 24 | 36 Wiener index | 3 | 9 Hosoya index | 4 | 4 Balaban index | 2.25 | 2.324
3D structure
3D structure
Basic properties
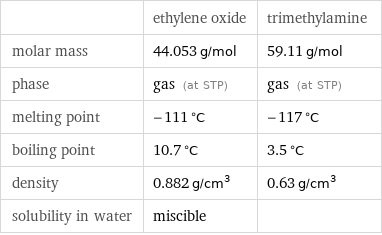
| ethylene oxide | trimethylamine molar mass | 44.053 g/mol | 59.11 g/mol phase | gas (at STP) | gas (at STP) melting point | -111 °C | -117 °C boiling point | 10.7 °C | 3.5 °C density | 0.882 g/cm^3 | 0.63 g/cm^3 solubility in water | miscible |
Units

Gas properties (at STP)
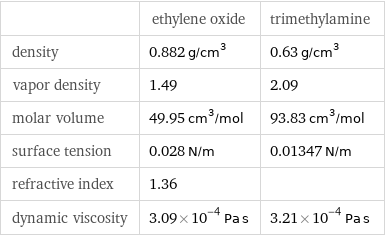
| ethylene oxide | trimethylamine density | 0.882 g/cm^3 | 0.63 g/cm^3 vapor density | 1.49 | 2.09 molar volume | 49.95 cm^3/mol | 93.83 cm^3/mol surface tension | 0.028 N/m | 0.01347 N/m refractive index | 1.36 | dynamic viscosity | 3.09×10^-4 Pa s | 3.21×10^-4 Pa s
Units

Thermodynamic properties
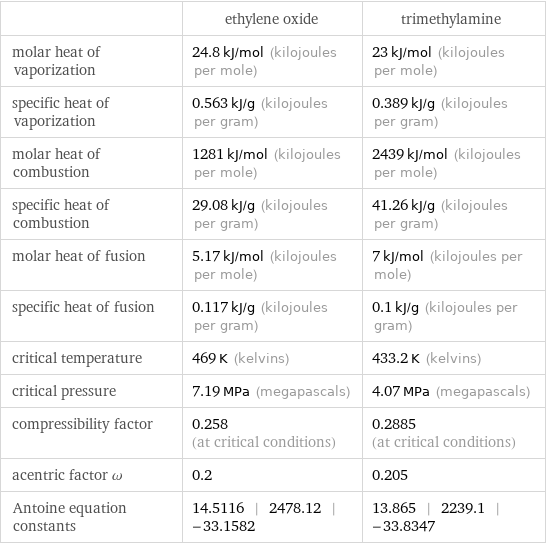
| ethylene oxide | trimethylamine molar heat of vaporization | 24.8 kJ/mol (kilojoules per mole) | 23 kJ/mol (kilojoules per mole) specific heat of vaporization | 0.563 kJ/g (kilojoules per gram) | 0.389 kJ/g (kilojoules per gram) molar heat of combustion | 1281 kJ/mol (kilojoules per mole) | 2439 kJ/mol (kilojoules per mole) specific heat of combustion | 29.08 kJ/g (kilojoules per gram) | 41.26 kJ/g (kilojoules per gram) molar heat of fusion | 5.17 kJ/mol (kilojoules per mole) | 7 kJ/mol (kilojoules per mole) specific heat of fusion | 0.117 kJ/g (kilojoules per gram) | 0.1 kJ/g (kilojoules per gram) critical temperature | 469 K (kelvins) | 433.2 K (kelvins) critical pressure | 7.19 MPa (megapascals) | 4.07 MPa (megapascals) compressibility factor | 0.258 (at critical conditions) | 0.2885 (at critical conditions) acentric factor ω | 0.2 | 0.205 Antoine equation constants | 14.5116 | 2478.12 | -33.1582 | 13.865 | 2239.1 | -33.8347
Chemical identifiers
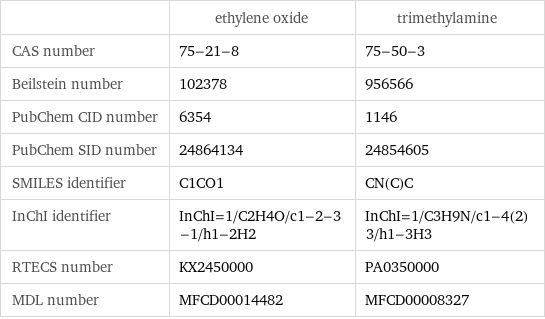
| ethylene oxide | trimethylamine CAS number | 75-21-8 | 75-50-3 Beilstein number | 102378 | 956566 PubChem CID number | 6354 | 1146 PubChem SID number | 24864134 | 24854605 SMILES identifier | C1CO1 | CN(C)C InChI identifier | InChI=1/C2H4O/c1-2-3-1/h1-2H2 | InChI=1/C3H9N/c1-4(2)3/h1-3H3 RTECS number | KX2450000 | PA0350000 MDL number | MFCD00014482 | MFCD00008327
NFPA label
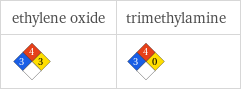
NFPA label
| ethylene oxide | trimethylamine NFPA health rating | 3 | 3 NFPA fire rating | 4 | 4 NFPA reactivity rating | 3 | 0
Safety properties
| ethylene oxide | trimethylamine flash point | -55 °C | -6.667 °C autoignition point | 429 °C | 190 °C lower explosive limit | 3% | 2% upper explosive limit | 100% | 11.6%
| ethylene oxide | trimethylamine DOT hazard class | 2.3 | 2.1 DOT numbers | 1040 | 1297
Toxicity properties
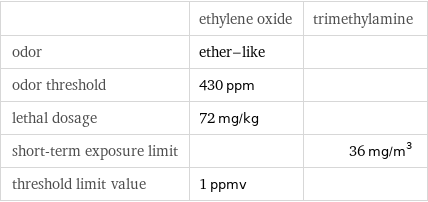
| ethylene oxide | trimethylamine odor | ether-like | odor threshold | 430 ppm | lethal dosage | 72 mg/kg | short-term exposure limit | | 36 mg/m^3 threshold limit value | 1 ppmv |
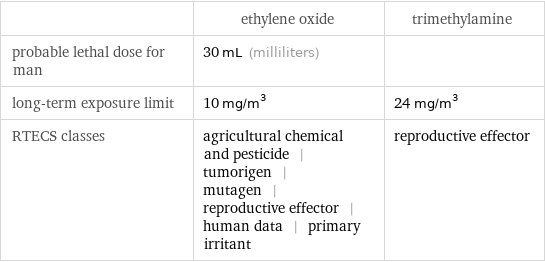
| ethylene oxide | trimethylamine probable lethal dose for man | 30 mL (milliliters) | long-term exposure limit | 10 mg/m^3 | 24 mg/m^3 RTECS classes | agricultural chemical and pesticide | tumorigen | mutagen | reproductive effector | human data | primary irritant | reproductive effector