Input interpretation
barium (chemical element) | strontium (chemical element) | holmium (chemical element)
Periodic table location
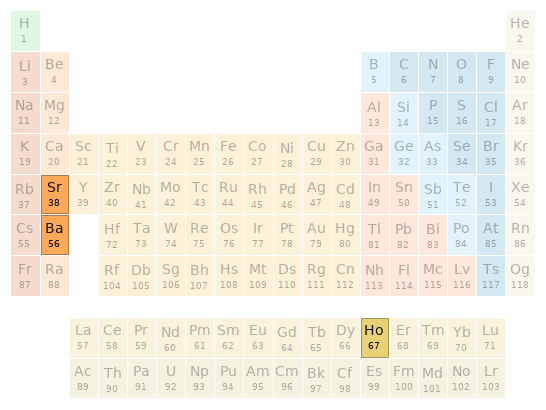
Periodic table location
Images

Images
Basic elemental properties
![| barium | strontium | holmium atomic symbol | Ba | Sr | Ho atomic number | 56 | 38 | 67 short electronic configuration | [Xe]6s^2 | [Kr]5s^2 | [Xe]6s^24f^11 Aufbau diagram | 6s | 5s | 4f 6s block | s | s | f group | 2 | 2 | period | 6 | 5 | 6 atomic mass | 137.327 u | 87.62 u | 164.93033 u half-life | (stable) | (stable) | (stable)](../image_source/ec6336bca369b778494d038fd654ea29.png)
| barium | strontium | holmium atomic symbol | Ba | Sr | Ho atomic number | 56 | 38 | 67 short electronic configuration | [Xe]6s^2 | [Kr]5s^2 | [Xe]6s^24f^11 Aufbau diagram | 6s | 5s | 4f 6s block | s | s | f group | 2 | 2 | period | 6 | 5 | 6 atomic mass | 137.327 u | 87.62 u | 164.93033 u half-life | (stable) | (stable) | (stable)
Thermodynamic properties
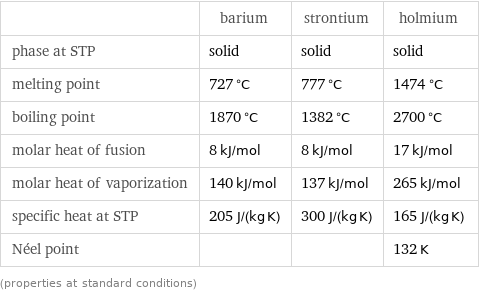
| barium | strontium | holmium phase at STP | solid | solid | solid melting point | 727 °C | 777 °C | 1474 °C boiling point | 1870 °C | 1382 °C | 2700 °C molar heat of fusion | 8 kJ/mol | 8 kJ/mol | 17 kJ/mol molar heat of vaporization | 140 kJ/mol | 137 kJ/mol | 265 kJ/mol specific heat at STP | 205 J/(kg K) | 300 J/(kg K) | 165 J/(kg K) Néel point | | | 132 K (properties at standard conditions)
Material properties
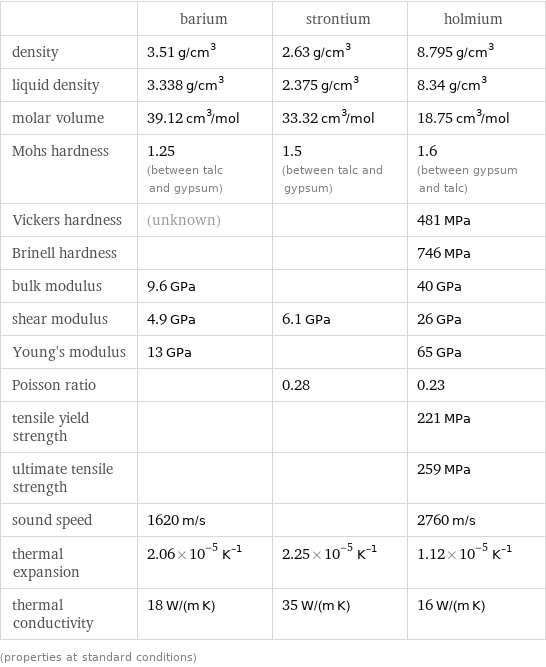
| barium | strontium | holmium density | 3.51 g/cm^3 | 2.63 g/cm^3 | 8.795 g/cm^3 liquid density | 3.338 g/cm^3 | 2.375 g/cm^3 | 8.34 g/cm^3 molar volume | 39.12 cm^3/mol | 33.32 cm^3/mol | 18.75 cm^3/mol Mohs hardness | 1.25 (between talc and gypsum) | 1.5 (between talc and gypsum) | 1.6 (between gypsum and talc) Vickers hardness | (unknown) | | 481 MPa Brinell hardness | | | 746 MPa bulk modulus | 9.6 GPa | | 40 GPa shear modulus | 4.9 GPa | 6.1 GPa | 26 GPa Young's modulus | 13 GPa | | 65 GPa Poisson ratio | | 0.28 | 0.23 tensile yield strength | | | 221 MPa ultimate tensile strength | | | 259 MPa sound speed | 1620 m/s | | 2760 m/s thermal expansion | 2.06×10^-5 K^(-1) | 2.25×10^-5 K^(-1) | 1.12×10^-5 K^(-1) thermal conductivity | 18 W/(m K) | 35 W/(m K) | 16 W/(m K) (properties at standard conditions)
Electromagnetic properties
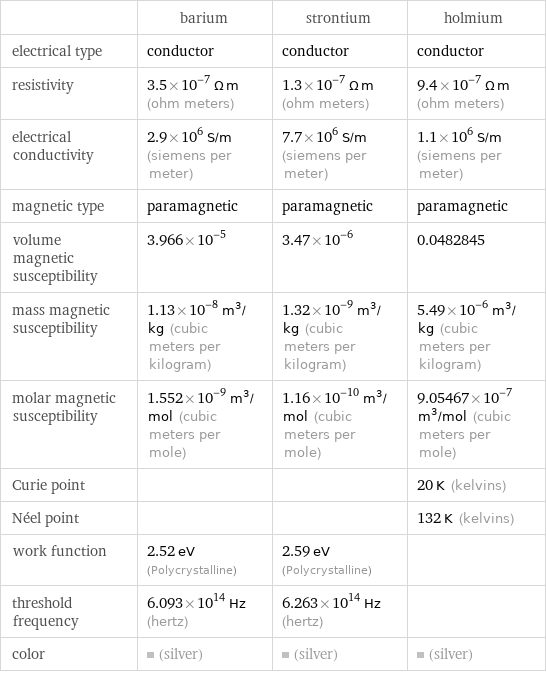
| barium | strontium | holmium electrical type | conductor | conductor | conductor resistivity | 3.5×10^-7 Ω m (ohm meters) | 1.3×10^-7 Ω m (ohm meters) | 9.4×10^-7 Ω m (ohm meters) electrical conductivity | 2.9×10^6 S/m (siemens per meter) | 7.7×10^6 S/m (siemens per meter) | 1.1×10^6 S/m (siemens per meter) magnetic type | paramagnetic | paramagnetic | paramagnetic volume magnetic susceptibility | 3.966×10^-5 | 3.47×10^-6 | 0.0482845 mass magnetic susceptibility | 1.13×10^-8 m^3/kg (cubic meters per kilogram) | 1.32×10^-9 m^3/kg (cubic meters per kilogram) | 5.49×10^-6 m^3/kg (cubic meters per kilogram) molar magnetic susceptibility | 1.552×10^-9 m^3/mol (cubic meters per mole) | 1.16×10^-10 m^3/mol (cubic meters per mole) | 9.05467×10^-7 m^3/mol (cubic meters per mole) Curie point | | | 20 K (kelvins) Néel point | | | 132 K (kelvins) work function | 2.52 eV (Polycrystalline) | 2.59 eV (Polycrystalline) | threshold frequency | 6.093×10^14 Hz (hertz) | 6.263×10^14 Hz (hertz) | color | (silver) | (silver) | (silver)
Reactivity
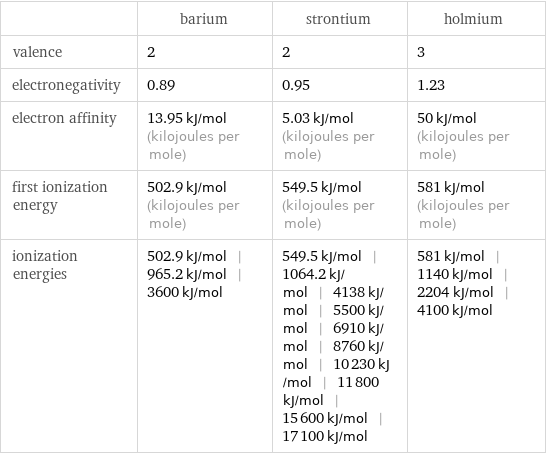
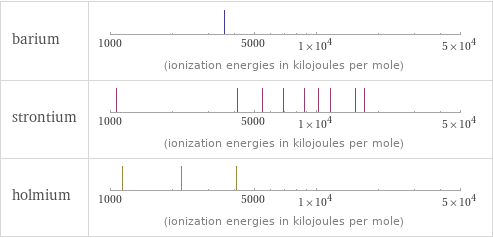
| barium | strontium | holmium valence | 2 | 2 | 3 electronegativity | 0.89 | 0.95 | 1.23 electron affinity | 13.95 kJ/mol (kilojoules per mole) | 5.03 kJ/mol (kilojoules per mole) | 50 kJ/mol (kilojoules per mole) first ionization energy | 502.9 kJ/mol (kilojoules per mole) | 549.5 kJ/mol (kilojoules per mole) | 581 kJ/mol (kilojoules per mole) ionization energies | 502.9 kJ/mol | 965.2 kJ/mol | 3600 kJ/mol | 549.5 kJ/mol | 1064.2 kJ/mol | 4138 kJ/mol | 5500 kJ/mol | 6910 kJ/mol | 8760 kJ/mol | 10230 kJ/mol | 11800 kJ/mol | 15600 kJ/mol | 17100 kJ/mol | 581 kJ/mol | 1140 kJ/mol | 2204 kJ/mol | 4100 kJ/mol
Reactivity
Atomic properties
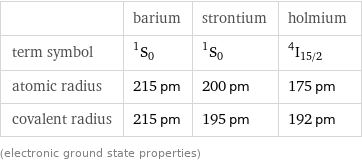
| barium | strontium | holmium term symbol | ^1S_0 | ^1S_0 | ^4I_(15/2) atomic radius | 215 pm | 200 pm | 175 pm covalent radius | 215 pm | 195 pm | 192 pm (electronic ground state properties)
Abundances
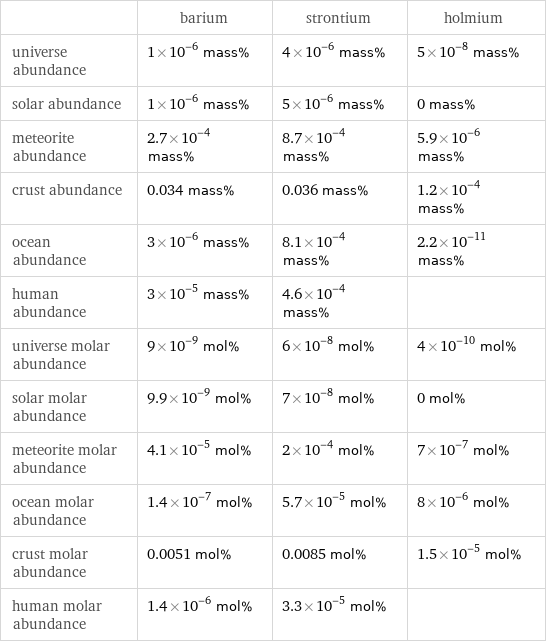
| barium | strontium | holmium universe abundance | 1×10^-6 mass% | 4×10^-6 mass% | 5×10^-8 mass% solar abundance | 1×10^-6 mass% | 5×10^-6 mass% | 0 mass% meteorite abundance | 2.7×10^-4 mass% | 8.7×10^-4 mass% | 5.9×10^-6 mass% crust abundance | 0.034 mass% | 0.036 mass% | 1.2×10^-4 mass% ocean abundance | 3×10^-6 mass% | 8.1×10^-4 mass% | 2.2×10^-11 mass% human abundance | 3×10^-5 mass% | 4.6×10^-4 mass% | universe molar abundance | 9×10^-9 mol% | 6×10^-8 mol% | 4×10^-10 mol% solar molar abundance | 9.9×10^-9 mol% | 7×10^-8 mol% | 0 mol% meteorite molar abundance | 4.1×10^-5 mol% | 2×10^-4 mol% | 7×10^-7 mol% ocean molar abundance | 1.4×10^-7 mol% | 5.7×10^-5 mol% | 8×10^-6 mol% crust molar abundance | 0.0051 mol% | 0.0085 mol% | 1.5×10^-5 mol% human molar abundance | 1.4×10^-6 mol% | 3.3×10^-5 mol% |
Nuclear properties
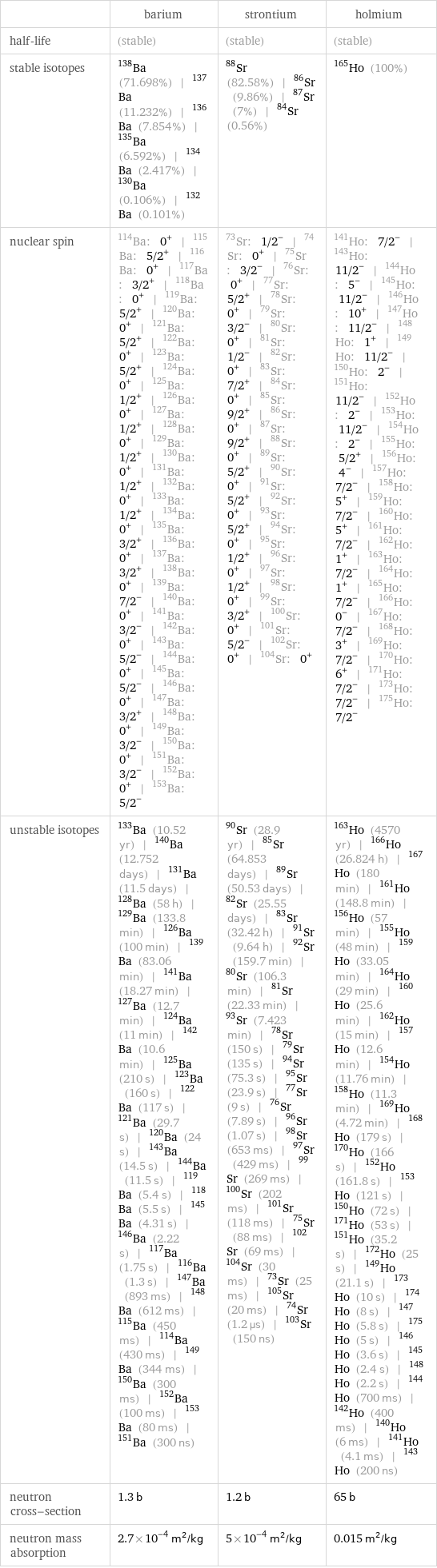
| barium | strontium | holmium half-life | (stable) | (stable) | (stable) stable isotopes | Ba-138 (71.698%) | Ba-137 (11.232%) | Ba-136 (7.854%) | Ba-135 (6.592%) | Ba-134 (2.417%) | Ba-130 (0.106%) | Ba-132 (0.101%) | Sr-88 (82.58%) | Sr-86 (9.86%) | Sr-87 (7%) | Sr-84 (0.56%) | Ho-165 (100%) nuclear spin | Ba-114: 0^+ | Ba-115: 5/2^+ | Ba-116: 0^+ | Ba-117: 3/2^+ | Ba-118: 0^+ | Ba-119: 5/2^+ | Ba-120: 0^+ | Ba-121: 5/2^+ | Ba-122: 0^+ | Ba-123: 5/2^+ | Ba-124: 0^+ | Ba-125: 1/2^+ | Ba-126: 0^+ | Ba-127: 1/2^+ | Ba-128: 0^+ | Ba-129: 1/2^+ | Ba-130: 0^+ | Ba-131: 1/2^+ | Ba-132: 0^+ | Ba-133: 1/2^+ | Ba-134: 0^+ | Ba-135: 3/2^+ | Ba-136: 0^+ | Ba-137: 3/2^+ | Ba-138: 0^+ | Ba-139: 7/2^- | Ba-140: 0^+ | Ba-141: 3/2^- | Ba-142: 0^+ | Ba-143: 5/2^- | Ba-144: 0^+ | Ba-145: 5/2^- | Ba-146: 0^+ | Ba-147: 3/2^+ | Ba-148: 0^+ | Ba-149: 3/2^- | Ba-150: 0^+ | Ba-151: 3/2^- | Ba-152: 0^+ | Ba-153: 5/2^- | Sr-73: 1/2^- | Sr-74: 0^+ | Sr-75: 3/2^- | Sr-76: 0^+ | Sr-77: 5/2^+ | Sr-78: 0^+ | Sr-79: 3/2^- | Sr-80: 0^+ | Sr-81: 1/2^- | Sr-82: 0^+ | Sr-83: 7/2^+ | Sr-84: 0^+ | Sr-85: 9/2^+ | Sr-86: 0^+ | Sr-87: 9/2^+ | Sr-88: 0^+ | Sr-89: 5/2^+ | Sr-90: 0^+ | Sr-91: 5/2^+ | Sr-92: 0^+ | Sr-93: 5/2^+ | Sr-94: 0^+ | Sr-95: 1/2^+ | Sr-96: 0^+ | Sr-97: 1/2^+ | Sr-98: 0^+ | Sr-99: 3/2^+ | Sr-100: 0^+ | Sr-101: 5/2^- | Sr-102: 0^+ | Sr-104: 0^+ | Ho-141: 7/2^- | Ho-143: 11/2^- | Ho-144: 5^- | Ho-145: 11/2^- | Ho-146: 10^+ | Ho-147: 11/2^- | Ho-148: 1^+ | Ho-149: 11/2^- | Ho-150: 2^- | Ho-151: 11/2^- | Ho-152: 2^- | Ho-153: 11/2^- | Ho-154: 2^- | Ho-155: 5/2^+ | Ho-156: 4^- | Ho-157: 7/2^- | Ho-158: 5^+ | Ho-159: 7/2^- | Ho-160: 5^+ | Ho-161: 7/2^- | Ho-162: 1^+ | Ho-163: 7/2^- | Ho-164: 1^+ | Ho-165: 7/2^- | Ho-166: 0^- | Ho-167: 7/2^- | Ho-168: 3^+ | Ho-169: 7/2^- | Ho-170: 6^+ | Ho-171: 7/2^- | Ho-173: 7/2^- | Ho-175: 7/2^- unstable isotopes | Ba-133 (10.52 yr) | Ba-140 (12.752 days) | Ba-131 (11.5 days) | Ba-128 (58 h) | Ba-129 (133.8 min) | Ba-126 (100 min) | Ba-139 (83.06 min) | Ba-141 (18.27 min) | Ba-127 (12.7 min) | Ba-124 (11 min) | Ba-142 (10.6 min) | Ba-125 (210 s) | Ba-123 (160 s) | Ba-122 (117 s) | Ba-121 (29.7 s) | Ba-120 (24 s) | Ba-143 (14.5 s) | Ba-144 (11.5 s) | Ba-119 (5.4 s) | Ba-118 (5.5 s) | Ba-145 (4.31 s) | Ba-146 (2.22 s) | Ba-117 (1.75 s) | Ba-116 (1.3 s) | Ba-147 (893 ms) | Ba-148 (612 ms) | Ba-115 (450 ms) | Ba-114 (430 ms) | Ba-149 (344 ms) | Ba-150 (300 ms) | Ba-152 (100 ms) | Ba-153 (80 ms) | Ba-151 (300 ns) | Sr-90 (28.9 yr) | Sr-85 (64.853 days) | Sr-89 (50.53 days) | Sr-82 (25.55 days) | Sr-83 (32.42 h) | Sr-91 (9.64 h) | Sr-92 (159.7 min) | Sr-80 (106.3 min) | Sr-81 (22.33 min) | Sr-93 (7.423 min) | Sr-78 (150 s) | Sr-79 (135 s) | Sr-94 (75.3 s) | Sr-95 (23.9 s) | Sr-77 (9 s) | Sr-76 (7.89 s) | Sr-96 (1.07 s) | Sr-98 (653 ms) | Sr-97 (429 ms) | Sr-99 (269 ms) | Sr-100 (202 ms) | Sr-101 (118 ms) | Sr-75 (88 ms) | Sr-102 (69 ms) | Sr-104 (30 ms) | Sr-73 (25 ms) | Sr-105 (20 ms) | Sr-74 (1.2 µs) | Sr-103 (150 ns) | Ho-163 (4570 yr) | Ho-166 (26.824 h) | Ho-167 (180 min) | Ho-161 (148.8 min) | Ho-156 (57 min) | Ho-155 (48 min) | Ho-159 (33.05 min) | Ho-164 (29 min) | Ho-160 (25.6 min) | Ho-162 (15 min) | Ho-157 (12.6 min) | Ho-154 (11.76 min) | Ho-158 (11.3 min) | Ho-169 (4.72 min) | Ho-168 (179 s) | Ho-170 (166 s) | Ho-152 (161.8 s) | Ho-153 (121 s) | Ho-150 (72 s) | Ho-171 (53 s) | Ho-151 (35.2 s) | Ho-172 (25 s) | Ho-149 (21.1 s) | Ho-173 (10 s) | Ho-174 (8 s) | Ho-147 (5.8 s) | Ho-175 (5 s) | Ho-146 (3.6 s) | Ho-145 (2.4 s) | Ho-148 (2.2 s) | Ho-144 (700 ms) | Ho-142 (400 ms) | Ho-140 (6 ms) | Ho-141 (4.1 ms) | Ho-143 (200 ns) neutron cross-section | 1.3 b | 1.2 b | 65 b neutron mass absorption | 2.7×10^-4 m^2/kg | 5×10^-4 m^2/kg | 0.015 m^2/kg