Input interpretation
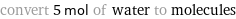
convert 5 mol of water to molecules
Results
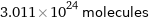
3.011×10^24 molecules
Possible intermediate steps
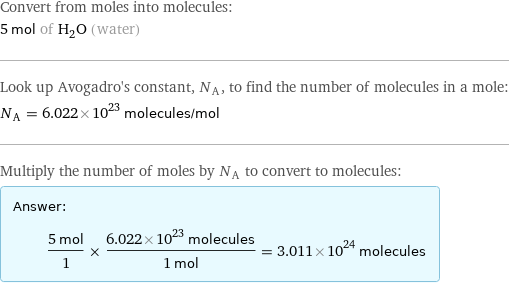
Convert from moles into molecules: 5 mol of H_2O (water) Look up Avogadro's constant, N_A, to find the number of molecules in a mole: N_A = 6.022×10^23 molecules/mol Multiply the number of moles by N_A to convert to molecules: Answer: | | (5 mol)/1 × (6.022×10^23 molecules)/(1 mol) = 3.011×10^24 molecules
Interpretation
molecule count
Basic unit dimensions
![[molecule]](../image_source/a1e7fed8d713a30157348256839d952c.png)
[molecule]
Corresponding quantity
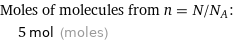
Moles of molecules from n = N/N_A: | 5 mol (moles)