Input interpretation
sodium bromide
Chemical names and formulas

formula | NaBr Hill formula | BrNa name | sodium bromide alternate names | bromide salt of sodium | hydrobromic acid sodium salt | sedoneural mass fractions | Br (bromine) 77.7% | Na (sodium) 22.3%
Structure diagram
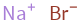
Structure diagram
Basic properties
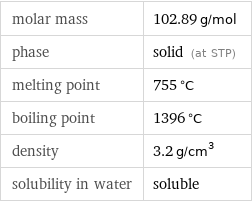
molar mass | 102.89 g/mol phase | solid (at STP) melting point | 755 °C boiling point | 1396 °C density | 3.2 g/cm^3 solubility in water | soluble
Units

Solid properties (at STP)
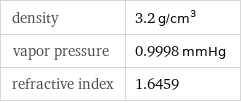
density | 3.2 g/cm^3 vapor pressure | 0.9998 mmHg refractive index | 1.6459
Units

Thermodynamic properties
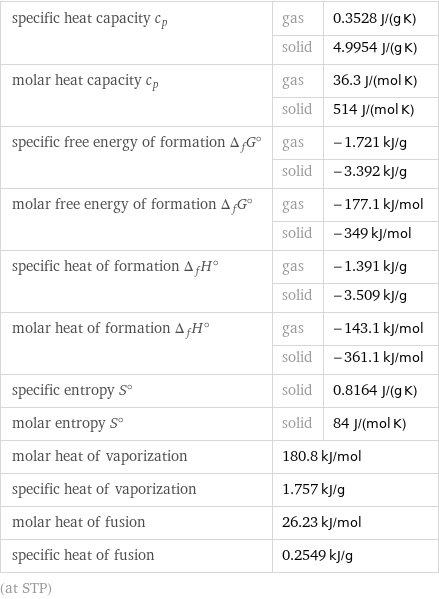
specific heat capacity c_p | gas | 0.3528 J/(g K) | solid | 4.9954 J/(g K) molar heat capacity c_p | gas | 36.3 J/(mol K) | solid | 514 J/(mol K) specific free energy of formation Δ_fG° | gas | -1.721 kJ/g | solid | -3.392 kJ/g molar free energy of formation Δ_fG° | gas | -177.1 kJ/mol | solid | -349 kJ/mol specific heat of formation Δ_fH° | gas | -1.391 kJ/g | solid | -3.509 kJ/g molar heat of formation Δ_fH° | gas | -143.1 kJ/mol | solid | -361.1 kJ/mol specific entropy S° | solid | 0.8164 J/(g K) molar entropy S° | solid | 84 J/(mol K) molar heat of vaporization | 180.8 kJ/mol | specific heat of vaporization | 1.757 kJ/g | molar heat of fusion | 26.23 kJ/mol | specific heat of fusion | 0.2549 kJ/g | (at STP)
Chemical identifiers
![CAS number | 7647-15-6 PubChem CID number | 253881 PubChem SID number | 24853756 SMILES identifier | [Na+].[Br-] InChI identifier | InChI=1/BrH.Na/h1H;/q;+1/p-1/fBr.Na/h1h;/q-1;m RTECS number | VZ3150000 MDL number | MFCD00003475](../image_source/85c85bb31d7eb42ca7cf68d9ed3f423c.png)
CAS number | 7647-15-6 PubChem CID number | 253881 PubChem SID number | 24853756 SMILES identifier | [Na+].[Br-] InChI identifier | InChI=1/BrH.Na/h1H;/q;+1/p-1/fBr.Na/h1h;/q-1;m RTECS number | VZ3150000 MDL number | MFCD00003475
Safety properties

flash point | 800 °C
Toxicity properties

lethal dosage | 3500 mg/kg (oral dose for rats)
Units

Ion equivalents

Na^+ (sodium cation) | 1 Br^- (bromide anion) | 1