Input interpretation
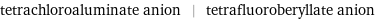
tetrachloroaluminate anion | tetrafluoroberyllate anion
General properties
![| tetrachloroaluminate anion | tetrafluoroberyllate anion formula | ([AlCl_4])^- | ([BeF_4])^(2-) net ionic charge | -1 | -2 alternate names | tetrachloroaluminate | tetrachloroaluminate(1-) | tetrachloroaluminate(III) | aluminium tetrachloride anion | tetrachloroaluminium anion | tetrafluoroberyllate | tetrafluoroberyllate(2-) | tetrafluoroberyllate(II)](../image_source/fa28fe06df260e5a9f5b66b57fdd2b6c.png)
| tetrachloroaluminate anion | tetrafluoroberyllate anion formula | ([AlCl_4])^- | ([BeF_4])^(2-) net ionic charge | -1 | -2 alternate names | tetrachloroaluminate | tetrachloroaluminate(1-) | tetrachloroaluminate(III) | aluminium tetrachloride anion | tetrachloroaluminium anion | tetrafluoroberyllate | tetrafluoroberyllate(2-) | tetrafluoroberyllate(II)
Ionic radii

| tetrachloroaluminate anion | tetrafluoroberyllate anion thermochemical radius | 295 pm | 245 pm
Units

Other properties

| tetrachloroaluminate anion | tetrafluoroberyllate anion ion class | anions | complex ions | metal halide ion | anions | complex ions | metal halide ion