Input interpretation
potassium chloride | 1, 1, 2, 2-tetrafluoroethane
Chemical names and formulas
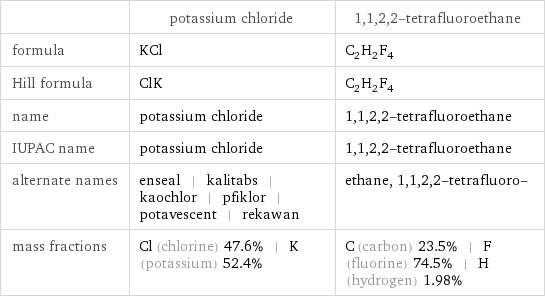
| potassium chloride | 1, 1, 2, 2-tetrafluoroethane formula | KCl | C_2H_2F_4 Hill formula | ClK | C_2H_2F_4 name | potassium chloride | 1, 1, 2, 2-tetrafluoroethane IUPAC name | potassium chloride | 1, 1, 2, 2-tetrafluoroethane alternate names | enseal | kalitabs | kaochlor | pfiklor | potavescent | rekawan | ethane, 1, 1, 2, 2-tetrafluoro- mass fractions | Cl (chlorine) 47.6% | K (potassium) 52.4% | C (carbon) 23.5% | F (fluorine) 74.5% | H (hydrogen) 1.98%
Structure diagrams
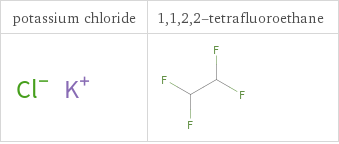
Structure diagrams
3D structure
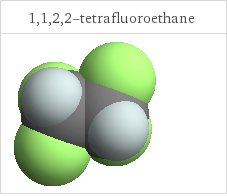
3D structure
Basic properties
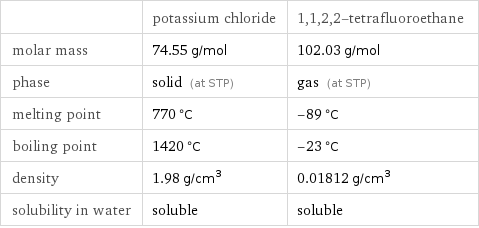
| potassium chloride | 1, 1, 2, 2-tetrafluoroethane molar mass | 74.55 g/mol | 102.03 g/mol phase | solid (at STP) | gas (at STP) melting point | 770 °C | -89 °C boiling point | 1420 °C | -23 °C density | 1.98 g/cm^3 | 0.01812 g/cm^3 solubility in water | soluble | soluble
Units
