Input interpretation
calcium iodide | trifluoroiodomethane
Chemical names and formulas
| calcium iodide | trifluoroiodomethane formula | CaI_2 | CF_3I Hill formula | CaI_2 | CF_3I name | calcium iodide | trifluoroiodomethane IUPAC name | calcium diiodide | trifluoro-iodomethane alternate names | calcium diiodide | calcium iodideanhydrous | yokaron | iodotrifluoromethane | monoiodotrifluoromethane | perfluoromethyl iodide | trifluoro-iodo-methane | trifluoro-iodomethane | trifluoromethyl iodide mass fractions | Ca (calcium) 13.6% | I (iodine) 86.4% | C (carbon) 6.13% | F (fluorine) 29.1% | I (iodine) 64.8%
Structure diagrams
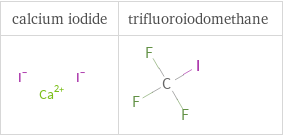
Structure diagrams
3D structure
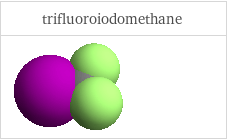
3D structure
Basic properties
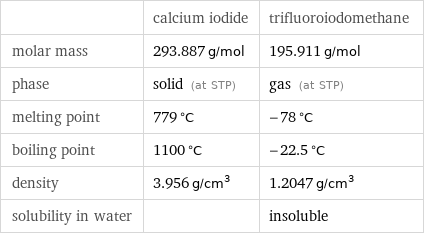
| calcium iodide | trifluoroiodomethane molar mass | 293.887 g/mol | 195.911 g/mol phase | solid (at STP) | gas (at STP) melting point | 779 °C | -78 °C boiling point | 1100 °C | -22.5 °C density | 3.956 g/cm^3 | 1.2047 g/cm^3 solubility in water | | insoluble
Units

Gas properties
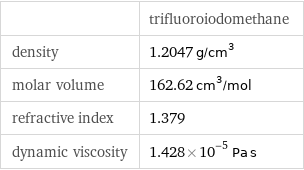
| trifluoroiodomethane density | 1.2047 g/cm^3 molar volume | 162.62 cm^3/mol refractive index | 1.379 dynamic viscosity | 1.428×10^-5 Pa s
Units
Thermodynamic properties
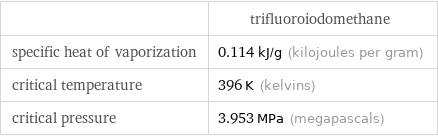
| trifluoroiodomethane specific heat of vaporization | 0.114 kJ/g (kilojoules per gram) critical temperature | 396 K (kelvins) critical pressure | 3.953 MPa (megapascals)