Input interpretation
Lewis bases | molar heat of combustion
Summary
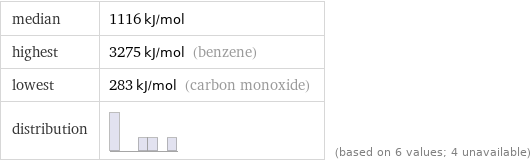
median | 1116 kJ/mol highest | 3275 kJ/mol (benzene) lowest | 283 kJ/mol (carbon monoxide) distribution | | (based on 6 values; 4 unavailable)
Entities with missing values
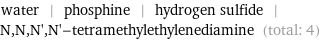
water | phosphine | hydrogen sulfide | N, N, N', N'-tetramethylethylenediamine (total: 4)
Molar heat of combustion rankings
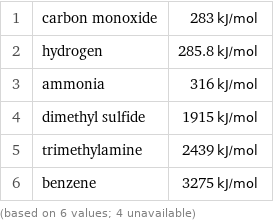
1 | carbon monoxide | 283 kJ/mol 2 | hydrogen | 285.8 kJ/mol 3 | ammonia | 316 kJ/mol 4 | dimethyl sulfide | 1915 kJ/mol 5 | trimethylamine | 2439 kJ/mol 6 | benzene | 3275 kJ/mol (based on 6 values; 4 unavailable)
Unit conversions for median molar heat of combustion 1116 kJ/mol
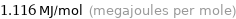
1.116 MJ/mol (megajoules per mole)
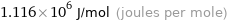
1.116×10^6 J/mol (joules per mole)
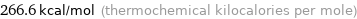
266.6 kcal/mol (thermochemical kilocalories per mole)

11.56 eV (molar electronvolts)
Corresponding quantity

Spectroscopic wavenumber ν^~: | 93250 cm^(-1) (reciprocal centimeters)