Input interpretation
potassium dihydrogen phosphate
Chemical names and formulas
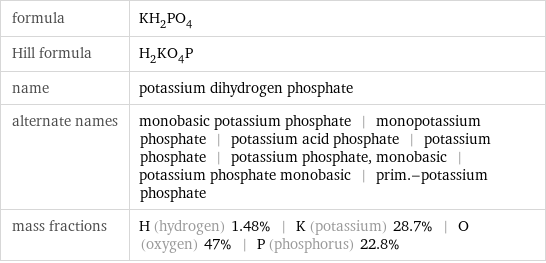
formula | KH_2PO_4 Hill formula | H_2KO_4P name | potassium dihydrogen phosphate alternate names | monobasic potassium phosphate | monopotassium phosphate | potassium acid phosphate | potassium phosphate | potassium phosphate, monobasic | potassium phosphate monobasic | prim.-potassium phosphate mass fractions | H (hydrogen) 1.48% | K (potassium) 28.7% | O (oxygen) 47% | P (phosphorus) 22.8%
Structure diagram
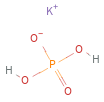
Structure diagram
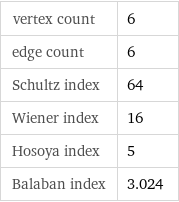
vertex count | 6 edge count | 6 Schultz index | 64 Wiener index | 16 Hosoya index | 5 Balaban index | 3.024
Basic properties
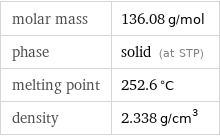
molar mass | 136.08 g/mol phase | solid (at STP) melting point | 252.6 °C density | 2.338 g/cm^3
Units

Solid properties (at STP)

density | 2.338 g/cm^3
Units

Thermodynamic properties
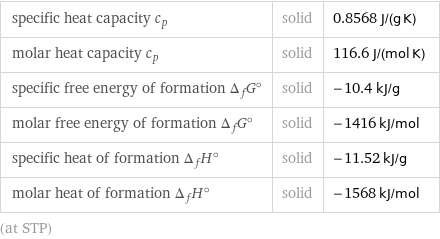
specific heat capacity c_p | solid | 0.8568 J/(g K) molar heat capacity c_p | solid | 116.6 J/(mol K) specific free energy of formation Δ_fG° | solid | -10.4 kJ/g molar free energy of formation Δ_fG° | solid | -1416 kJ/mol specific heat of formation Δ_fH° | solid | -11.52 kJ/g molar heat of formation Δ_fH° | solid | -1568 kJ/mol (at STP)
Chemical identifiers
![CAS number | 7778-77-0 PubChem CID number | 516951 SMILES identifier | OP(=O)(O)[O-].[K+] InChI identifier | InChI=1/K.H3O4P/c;1-5(2, 3)4/h;(H3, 1, 2, 3, 4)/q+1;/p-1/fK.H2O4P/h;1-2H/qm;-1 RTECS number | TC6615500 MDL number | MFCD00011401](../image_source/7532ca34ad713aef2a6eecffcdaebef5.png)
CAS number | 7778-77-0 PubChem CID number | 516951 SMILES identifier | OP(=O)(O)[O-].[K+] InChI identifier | InChI=1/K.H3O4P/c;1-5(2, 3)4/h;(H3, 1, 2, 3, 4)/q+1;/p-1/fK.H2O4P/h;1-2H/qm;-1 RTECS number | TC6615500 MDL number | MFCD00011401
NFPA label

NFPA label

NFPA health rating | 1 NFPA fire rating | 0 NFPA reactivity rating | 0
Toxicity properties

RTECS classes | other
Ion equivalents

K^+ (potassium cation) | 1 (H_2PO_4)^- (dihydrogen phosphate anion) | 1