Input interpretation

propane | orbital hybridization
Result
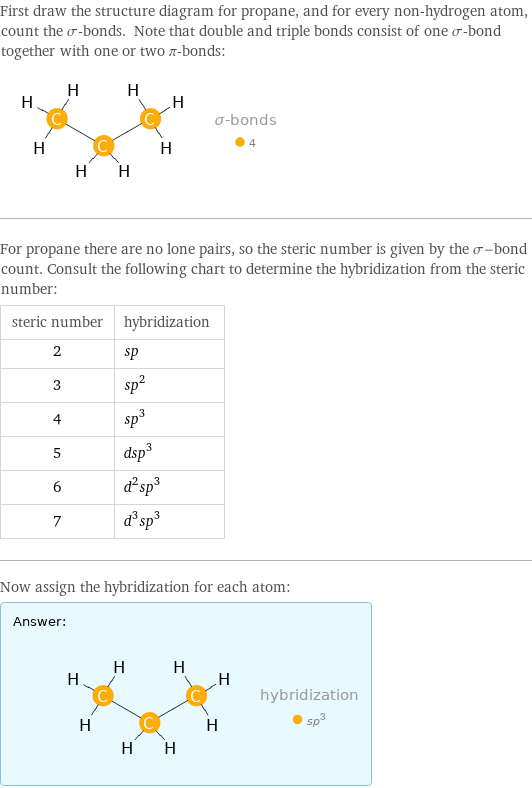
First draw the structure diagram for propane, and for every non-hydrogen atom, count the σ-bonds. Note that double and triple bonds consist of one σ-bond together with one or two π-bonds: For propane there are no lone pairs, so the steric number is given by the σ-bond count. Consult the following chart to determine the hybridization from the steric number: steric number | hybridization 2 | sp 3 | sp^2 4 | sp^3 5 | dsp^3 6 | d^2sp^3 7 | d^3sp^3 Now assign the hybridization for each atom: Answer: | |
Chemical names and formulas
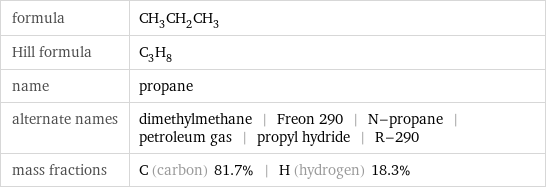
formula | CH_3CH_2CH_3 Hill formula | C_3H_8 name | propane alternate names | dimethylmethane | Freon 290 | N-propane | petroleum gas | propyl hydride | R-290 mass fractions | C (carbon) 81.7% | H (hydrogen) 18.3%