Input interpretation
nickel iodide
Chemical names and formulas
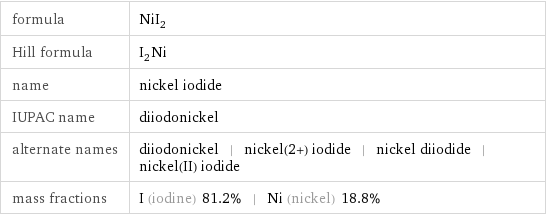
formula | NiI_2 Hill formula | I_2Ni name | nickel iodide IUPAC name | diiodonickel alternate names | diiodonickel | nickel(2+) iodide | nickel diiodide | nickel(II) iodide mass fractions | I (iodine) 81.2% | Ni (nickel) 18.8%
Structure diagram

Structure diagram
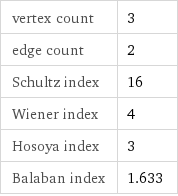
vertex count | 3 edge count | 2 Schultz index | 16 Wiener index | 4 Hosoya index | 3 Balaban index | 1.633
Basic properties
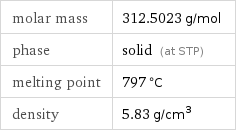
molar mass | 312.5023 g/mol phase | solid (at STP) melting point | 797 °C density | 5.83 g/cm^3
Units

Solid properties (at STP)

density | 5.83 g/cm^3
Units

Thermodynamic properties
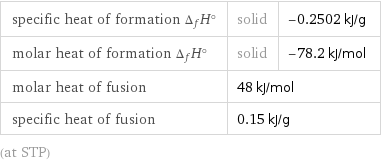
specific heat of formation Δ_fH° | solid | -0.2502 kJ/g molar heat of formation Δ_fH° | solid | -78.2 kJ/mol molar heat of fusion | 48 kJ/mol | specific heat of fusion | 0.15 kJ/g | (at STP)
Chemical identifiers
I InChI identifier | InChI=1/2HI.Ni/h2*1H;/q;;+2/p-2/f2I.Ni/h2*1h;/q2*-1;m MDL number | MFCD00016264](../image_source/ff6a0ad391ff04fd1b5ff8c2f5fb09c2.png)
CAS number | 13462-90-3 PubChem CID number | 26038 PubChem SID number | 24864951 SMILES identifier | [Ni](I)I InChI identifier | InChI=1/2HI.Ni/h2*1H;/q;;+2/p-2/f2I.Ni/h2*1h;/q2*-1;m MDL number | MFCD00016264