Input interpretation
helium (chemical element) | krypton (chemical element) | neon (chemical element)
Periodic table location
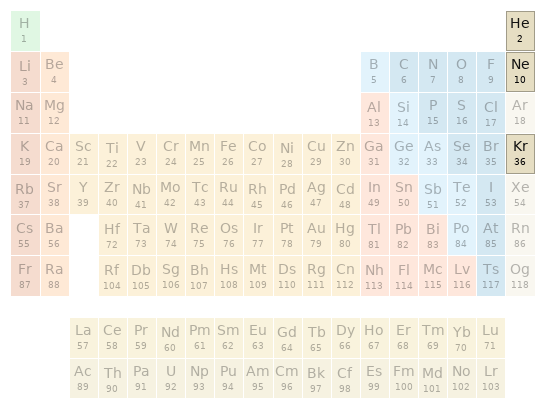
Periodic table location
Images
Images
Basic elemental properties
![| helium | krypton | neon atomic symbol | He | Kr | Ne atomic number | 2 | 36 | 10 short electronic configuration | 1s^2 | [Ar]4s^23d^104p^6 | [He]2s^22p^6 Aufbau diagram | 1s | 4p 3d 4s | 2p 2s block | s | p | p group | 18 | 18 | 18 period | 1 | 4 | 2 atomic mass | 4.002602 u | 83.798 u | 20.1797 u half-life | (stable) | (stable) | (stable)](../image_source/9aba68e93c7fe57711ddb3e56e79a864.png)
| helium | krypton | neon atomic symbol | He | Kr | Ne atomic number | 2 | 36 | 10 short electronic configuration | 1s^2 | [Ar]4s^23d^104p^6 | [He]2s^22p^6 Aufbau diagram | 1s | 4p 3d 4s | 2p 2s block | s | p | p group | 18 | 18 | 18 period | 1 | 4 | 2 atomic mass | 4.002602 u | 83.798 u | 20.1797 u half-life | (stable) | (stable) | (stable)
Thermodynamic properties
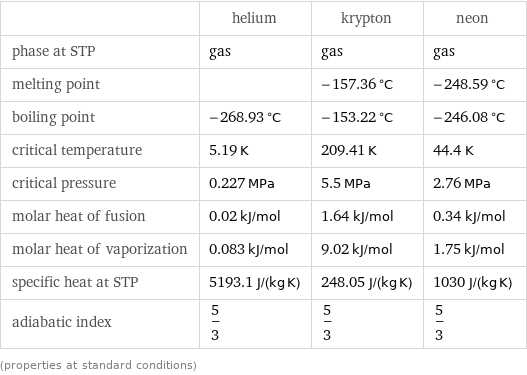
| helium | krypton | neon phase at STP | gas | gas | gas melting point | | -157.36 °C | -248.59 °C boiling point | -268.93 °C | -153.22 °C | -246.08 °C critical temperature | 5.19 K | 209.41 K | 44.4 K critical pressure | 0.227 MPa | 5.5 MPa | 2.76 MPa molar heat of fusion | 0.02 kJ/mol | 1.64 kJ/mol | 0.34 kJ/mol molar heat of vaporization | 0.083 kJ/mol | 9.02 kJ/mol | 1.75 kJ/mol specific heat at STP | 5193.1 J/(kg K) | 248.05 J/(kg K) | 1030 J/(kg K) adiabatic index | 5/3 | 5/3 | 5/3 (properties at standard conditions)
Material properties
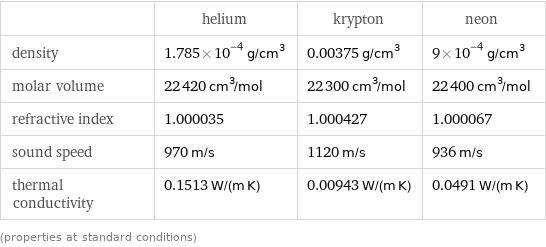
| helium | krypton | neon density | 1.785×10^-4 g/cm^3 | 0.00375 g/cm^3 | 9×10^-4 g/cm^3 molar volume | 22420 cm^3/mol | 22300 cm^3/mol | 22400 cm^3/mol refractive index | 1.000035 | 1.000427 | 1.000067 sound speed | 970 m/s | 1120 m/s | 936 m/s thermal conductivity | 0.1513 W/(m K) | 0.00943 W/(m K) | 0.0491 W/(m K) (properties at standard conditions)
Electromagnetic properties
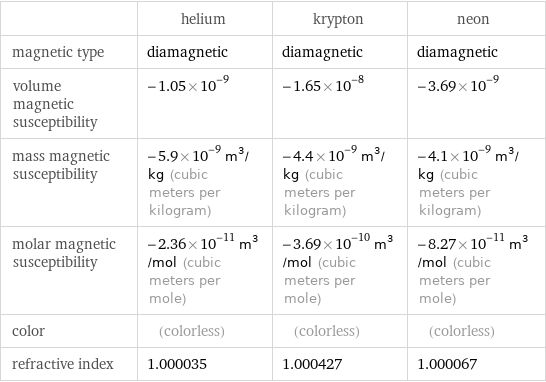
| helium | krypton | neon magnetic type | diamagnetic | diamagnetic | diamagnetic volume magnetic susceptibility | -1.05×10^-9 | -1.65×10^-8 | -3.69×10^-9 mass magnetic susceptibility | -5.9×10^-9 m^3/kg (cubic meters per kilogram) | -4.4×10^-9 m^3/kg (cubic meters per kilogram) | -4.1×10^-9 m^3/kg (cubic meters per kilogram) molar magnetic susceptibility | -2.36×10^-11 m^3/mol (cubic meters per mole) | -3.69×10^-10 m^3/mol (cubic meters per mole) | -8.27×10^-11 m^3/mol (cubic meters per mole) color | (colorless) | (colorless) | (colorless) refractive index | 1.000035 | 1.000427 | 1.000067
Reactivity
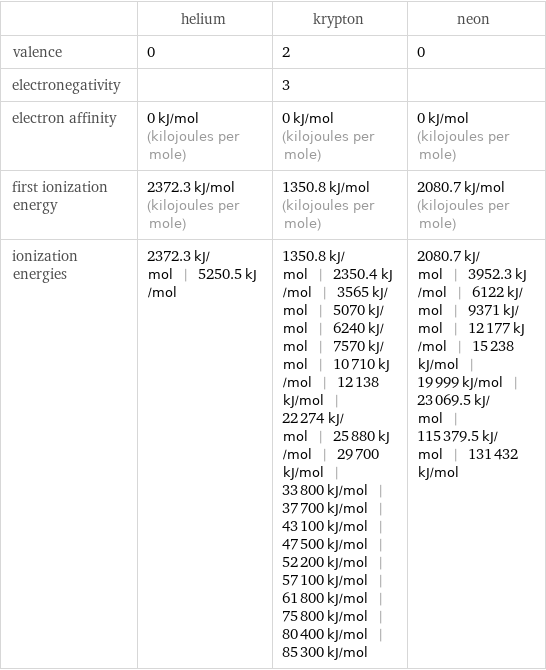
| helium | krypton | neon valence | 0 | 2 | 0 electronegativity | | 3 | electron affinity | 0 kJ/mol (kilojoules per mole) | 0 kJ/mol (kilojoules per mole) | 0 kJ/mol (kilojoules per mole) first ionization energy | 2372.3 kJ/mol (kilojoules per mole) | 1350.8 kJ/mol (kilojoules per mole) | 2080.7 kJ/mol (kilojoules per mole) ionization energies | 2372.3 kJ/mol | 5250.5 kJ/mol | 1350.8 kJ/mol | 2350.4 kJ/mol | 3565 kJ/mol | 5070 kJ/mol | 6240 kJ/mol | 7570 kJ/mol | 10710 kJ/mol | 12138 kJ/mol | 22274 kJ/mol | 25880 kJ/mol | 29700 kJ/mol | 33800 kJ/mol | 37700 kJ/mol | 43100 kJ/mol | 47500 kJ/mol | 52200 kJ/mol | 57100 kJ/mol | 61800 kJ/mol | 75800 kJ/mol | 80400 kJ/mol | 85300 kJ/mol | 2080.7 kJ/mol | 3952.3 kJ/mol | 6122 kJ/mol | 9371 kJ/mol | 12177 kJ/mol | 15238 kJ/mol | 19999 kJ/mol | 23069.5 kJ/mol | 115379.5 kJ/mol | 131432 kJ/mol
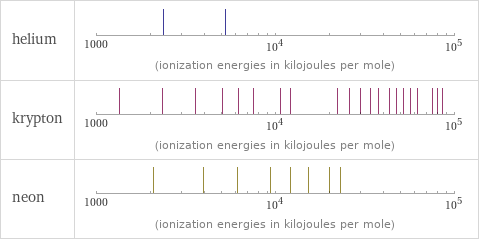
Reactivity
Atomic properties
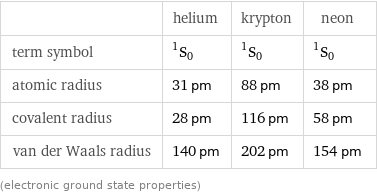
| helium | krypton | neon term symbol | ^1S_0 | ^1S_0 | ^1S_0 atomic radius | 31 pm | 88 pm | 38 pm covalent radius | 28 pm | 116 pm | 58 pm van der Waals radius | 140 pm | 202 pm | 154 pm (electronic ground state properties)
Abundances
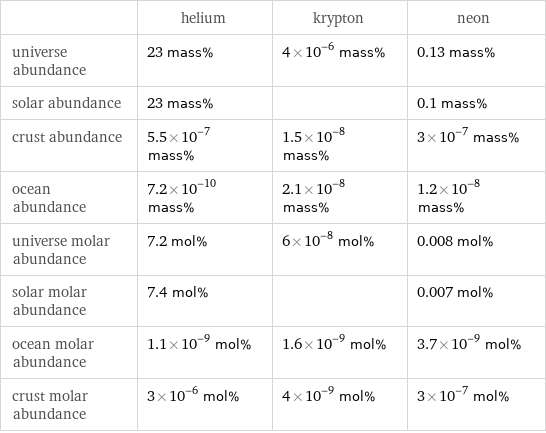
| helium | krypton | neon universe abundance | 23 mass% | 4×10^-6 mass% | 0.13 mass% solar abundance | 23 mass% | | 0.1 mass% crust abundance | 5.5×10^-7 mass% | 1.5×10^-8 mass% | 3×10^-7 mass% ocean abundance | 7.2×10^-10 mass% | 2.1×10^-8 mass% | 1.2×10^-8 mass% universe molar abundance | 7.2 mol% | 6×10^-8 mol% | 0.008 mol% solar molar abundance | 7.4 mol% | | 0.007 mol% ocean molar abundance | 1.1×10^-9 mol% | 1.6×10^-9 mol% | 3.7×10^-9 mol% crust molar abundance | 3×10^-6 mol% | 4×10^-9 mol% | 3×10^-7 mol%
Nuclear properties
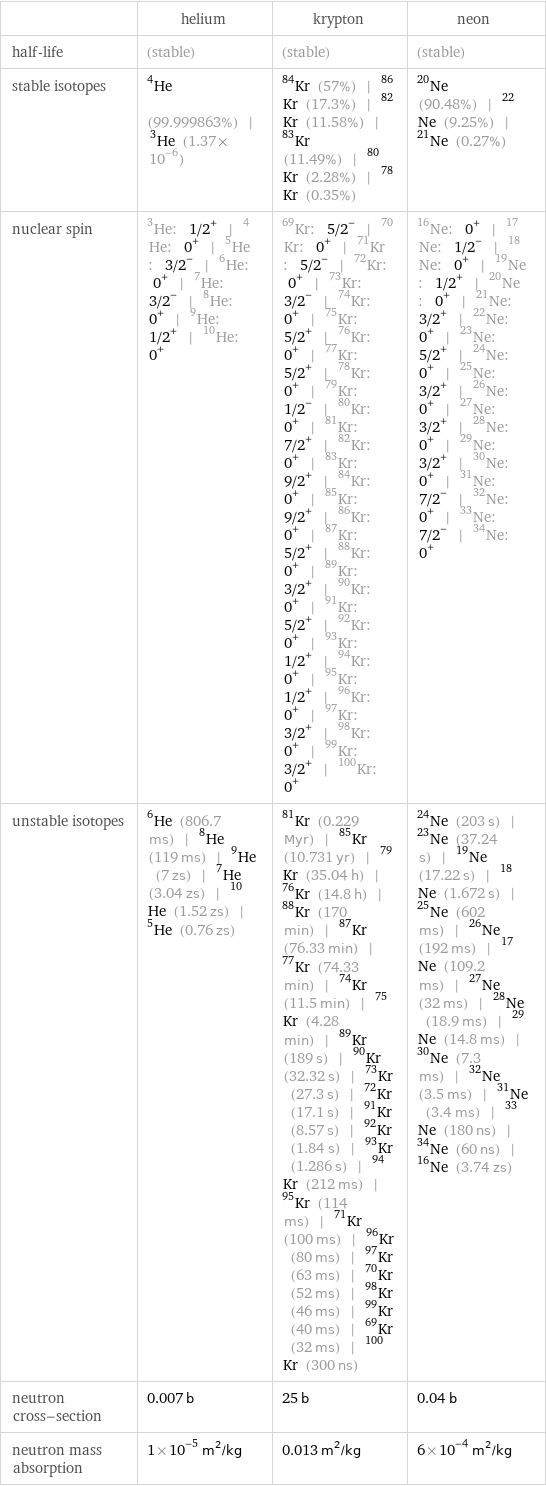
| helium | krypton | neon half-life | (stable) | (stable) | (stable) stable isotopes | He-4 (99.999863%) | He-3 (1.37×10^-6) | Kr-84 (57%) | Kr-86 (17.3%) | Kr-82 (11.58%) | Kr-83 (11.49%) | Kr-80 (2.28%) | Kr-78 (0.35%) | Ne-20 (90.48%) | Ne-22 (9.25%) | Ne-21 (0.27%) nuclear spin | He-3: 1/2^+ | He-4: 0^+ | He-5: 3/2^- | He-6: 0^+ | He-7: 3/2^- | He-8: 0^+ | He-9: 1/2^+ | He-10: 0^+ | Kr-69: 5/2^- | Kr-70: 0^+ | Kr-71: 5/2^- | Kr-72: 0^+ | Kr-73: 3/2^- | Kr-74: 0^+ | Kr-75: 5/2^+ | Kr-76: 0^+ | Kr-77: 5/2^+ | Kr-78: 0^+ | Kr-79: 1/2^- | Kr-80: 0^+ | Kr-81: 7/2^+ | Kr-82: 0^+ | Kr-83: 9/2^+ | Kr-84: 0^+ | Kr-85: 9/2^+ | Kr-86: 0^+ | Kr-87: 5/2^+ | Kr-88: 0^+ | Kr-89: 3/2^+ | Kr-90: 0^+ | Kr-91: 5/2^+ | Kr-92: 0^+ | Kr-93: 1/2^+ | Kr-94: 0^+ | Kr-95: 1/2^+ | Kr-96: 0^+ | Kr-97: 3/2^+ | Kr-98: 0^+ | Kr-99: 3/2^+ | Kr-100: 0^+ | Ne-16: 0^+ | Ne-17: 1/2^- | Ne-18: 0^+ | Ne-19: 1/2^+ | Ne-20: 0^+ | Ne-21: 3/2^+ | Ne-22: 0^+ | Ne-23: 5/2^+ | Ne-24: 0^+ | Ne-25: 3/2^+ | Ne-26: 0^+ | Ne-27: 3/2^+ | Ne-28: 0^+ | Ne-29: 3/2^+ | Ne-30: 0^+ | Ne-31: 7/2^- | Ne-32: 0^+ | Ne-33: 7/2^- | Ne-34: 0^+ unstable isotopes | He-6 (806.7 ms) | He-8 (119 ms) | He-9 (7 zs) | He-7 (3.04 zs) | He-10 (1.52 zs) | He-5 (0.76 zs) | Kr-81 (0.229 Myr) | Kr-85 (10.731 yr) | Kr-79 (35.04 h) | Kr-76 (14.8 h) | Kr-88 (170 min) | Kr-87 (76.33 min) | Kr-77 (74.33 min) | Kr-74 (11.5 min) | Kr-75 (4.28 min) | Kr-89 (189 s) | Kr-90 (32.32 s) | Kr-73 (27.3 s) | Kr-72 (17.1 s) | Kr-91 (8.57 s) | Kr-92 (1.84 s) | Kr-93 (1.286 s) | Kr-94 (212 ms) | Kr-95 (114 ms) | Kr-71 (100 ms) | Kr-96 (80 ms) | Kr-97 (63 ms) | Kr-70 (52 ms) | Kr-98 (46 ms) | Kr-99 (40 ms) | Kr-69 (32 ms) | Kr-100 (300 ns) | Ne-24 (203 s) | Ne-23 (37.24 s) | Ne-19 (17.22 s) | Ne-18 (1.672 s) | Ne-25 (602 ms) | Ne-26 (192 ms) | Ne-17 (109.2 ms) | Ne-27 (32 ms) | Ne-28 (18.9 ms) | Ne-29 (14.8 ms) | Ne-30 (7.3 ms) | Ne-32 (3.5 ms) | Ne-31 (3.4 ms) | Ne-33 (180 ns) | Ne-34 (60 ns) | Ne-16 (3.74 zs) neutron cross-section | 0.007 b | 25 b | 0.04 b neutron mass absorption | 1×10^-5 m^2/kg | 0.013 m^2/kg | 6×10^-4 m^2/kg