Input interpretation

Arrhenius bases | specific gas constant
Summary
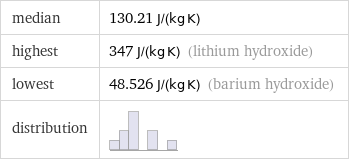
median | 130.21 J/(kg K) highest | 347 J/(kg K) (lithium hydroxide) lowest | 48.526 J/(kg K) (barium hydroxide) distribution |
Units

Distribution plots
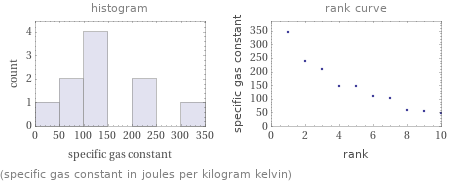
(specific gas constant in joules per kilogram kelvin)
Specific gas constant rankings
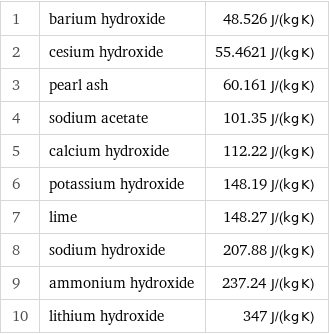
1 | barium hydroxide | 48.526 J/(kg K) 2 | cesium hydroxide | 55.4621 J/(kg K) 3 | pearl ash | 60.161 J/(kg K) 4 | sodium acetate | 101.35 J/(kg K) 5 | calcium hydroxide | 112.22 J/(kg K) 6 | potassium hydroxide | 148.19 J/(kg K) 7 | lime | 148.27 J/(kg K) 8 | sodium hydroxide | 207.88 J/(kg K) 9 | ammonium hydroxide | 237.24 J/(kg K) 10 | lithium hydroxide | 347 J/(kg K)
Unit conversions for median specific gas constant 130.21 J/(kg K)
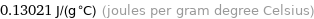
0.13021 J/(g °C) (joules per gram degree Celsius)

0.13021 J/(g K) (joules per gram kelvin)
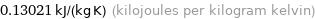
0.13021 kJ/(kg K) (kilojoules per kilogram kelvin)
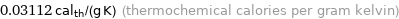
0.03112 cal_th/(g K) (thermochemical calories per gram kelvin)
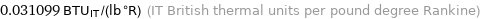
0.031099 BTU_IT/(lb °R) (IT British thermal units per pound degree Rankine)