Input interpretation
chromium(III) oxide | barium metaborate dihydrate
Chemical names and formulas
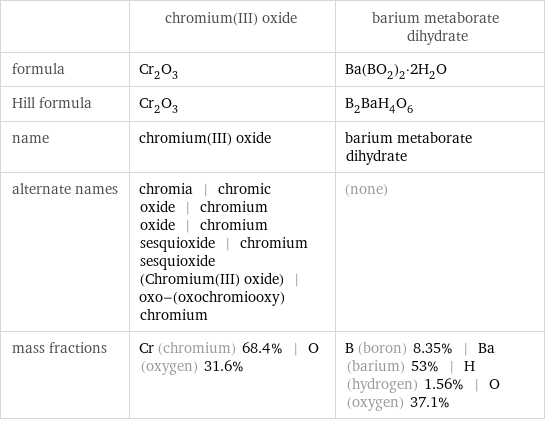
| chromium(III) oxide | barium metaborate dihydrate formula | Cr_2O_3 | Ba(BO_2)_2·2H_2O Hill formula | Cr_2O_3 | B_2BaH_4O_6 name | chromium(III) oxide | barium metaborate dihydrate alternate names | chromia | chromic oxide | chromium oxide | chromium sesquioxide | chromium sesquioxide (Chromium(III) oxide) | oxo-(oxochromiooxy)chromium | (none) mass fractions | Cr (chromium) 68.4% | O (oxygen) 31.6% | B (boron) 8.35% | Ba (barium) 53% | H (hydrogen) 1.56% | O (oxygen) 37.1%
Structure diagrams
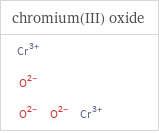
Structure diagrams
Basic properties
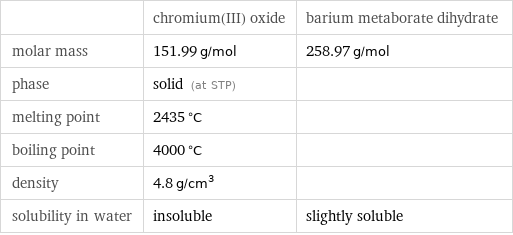
| chromium(III) oxide | barium metaborate dihydrate molar mass | 151.99 g/mol | 258.97 g/mol phase | solid (at STP) | melting point | 2435 °C | boiling point | 4000 °C | density | 4.8 g/cm^3 | solubility in water | insoluble | slightly soluble
Units
