Input interpretation
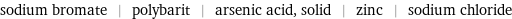
sodium bromate | polybarit | arsenic acid, solid | zinc | sodium chloride
Structure diagrams
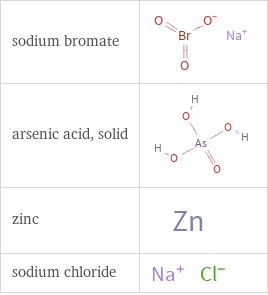
Structure diagrams
3D structure
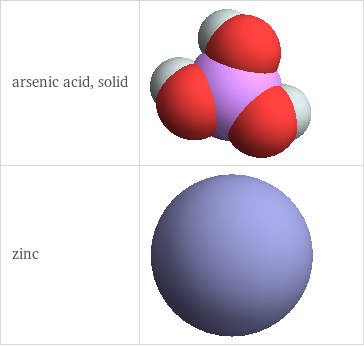
3D structure
Basic properties
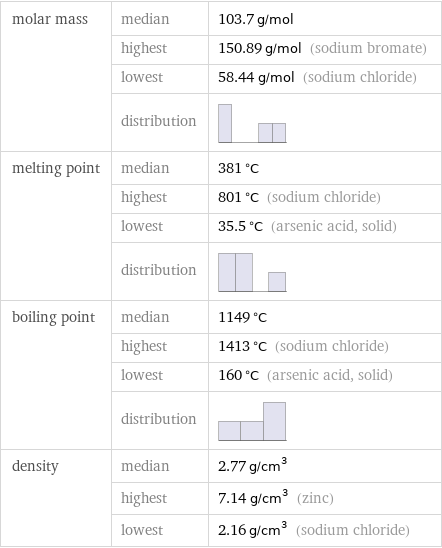
molar mass | median | 103.7 g/mol | highest | 150.89 g/mol (sodium bromate) | lowest | 58.44 g/mol (sodium chloride) | distribution | melting point | median | 381 °C | highest | 801 °C (sodium chloride) | lowest | 35.5 °C (arsenic acid, solid) | distribution | boiling point | median | 1149 °C | highest | 1413 °C (sodium chloride) | lowest | 160 °C (arsenic acid, solid) | distribution | density | median | 2.77 g/cm^3 | highest | 7.14 g/cm^3 (zinc) | lowest | 2.16 g/cm^3 (sodium chloride)
Units

NFPA label
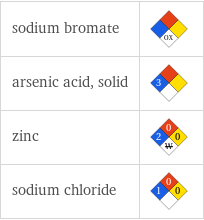
NFPA label