Input interpretation

HNO_3 nitric acid + Cu_2S copper(I) sulfide ⟶ H_2O water + NO_2 nitrogen dioxide + Cu(NO_3)_2 copper(II) nitrate + CuSO4SO4
Chemical names and formulas
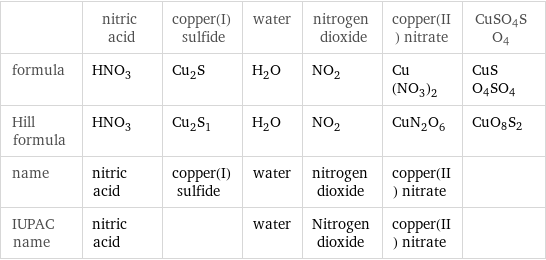
| nitric acid | copper(I) sulfide | water | nitrogen dioxide | copper(II) nitrate | CuSO4SO4 formula | HNO_3 | Cu_2S | H_2O | NO_2 | Cu(NO_3)_2 | CuSO4SO4 Hill formula | HNO_3 | Cu_2S_1 | H_2O | NO_2 | CuN_2O_6 | CuO8S2 name | nitric acid | copper(I) sulfide | water | nitrogen dioxide | copper(II) nitrate | IUPAC name | nitric acid | | water | Nitrogen dioxide | copper(II) nitrate |