Input interpretation

reductants | relative molecular mass
Summary
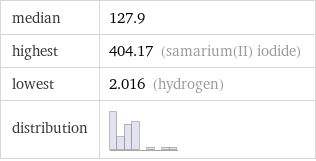
median | 127.9 highest | 404.17 (samarium(II) iodide) lowest | 2.016 (hydrogen) distribution |
Distribution plots
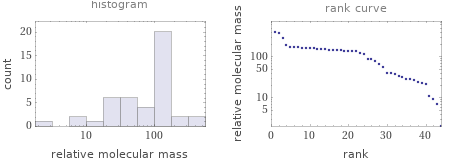
Relative molecular mass rankings
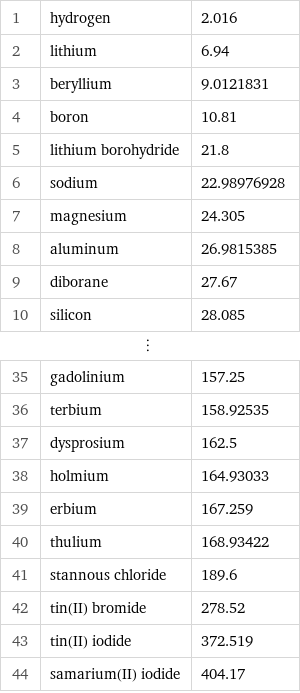
1 | hydrogen | 2.016 2 | lithium | 6.94 3 | beryllium | 9.0121831 4 | boron | 10.81 5 | lithium borohydride | 21.8 6 | sodium | 22.98976928 7 | magnesium | 24.305 8 | aluminum | 26.9815385 9 | diborane | 27.67 10 | silicon | 28.085 ⋮ | | 35 | gadolinium | 157.25 36 | terbium | 158.92535 37 | dysprosium | 162.5 38 | holmium | 164.93033 39 | erbium | 167.259 40 | thulium | 168.93422 41 | stannous chloride | 189.6 42 | tin(II) bromide | 278.52 43 | tin(II) iodide | 372.519 44 | samarium(II) iodide | 404.17
Comparisons for median relative molecular mass 127.9

≈ ( 0.18 ≈ 1/6 ) × relative molecular mass of fullerene ( ≈ 721 )

≈ 0.66 × relative molecular mass of caffeine ( ≈ 194 )
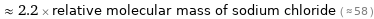
≈ 2.2 × relative molecular mass of sodium chloride ( ≈ 58 )
Corresponding quantities
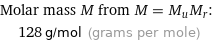
Molar mass M from M = M_uM_r: | 128 g/mol (grams per mole)

Molecular mass m from m = M_rM_u/N_A: | 2.1×10^-22 grams | 2.1×10^-25 kg (kilograms)