Input interpretation
lanthanum sulfide | beryllium oxide
Chemical names and formulas
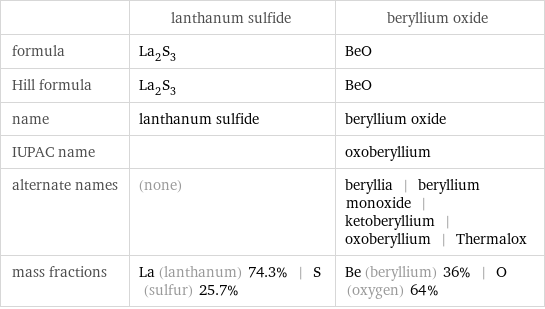
| lanthanum sulfide | beryllium oxide formula | La_2S_3 | BeO Hill formula | La_2S_3 | BeO name | lanthanum sulfide | beryllium oxide IUPAC name | | oxoberyllium alternate names | (none) | beryllia | beryllium monoxide | ketoberyllium | oxoberyllium | Thermalox mass fractions | La (lanthanum) 74.3% | S (sulfur) 25.7% | Be (beryllium) 36% | O (oxygen) 64%
Structure diagrams

Structure diagrams
Basic properties
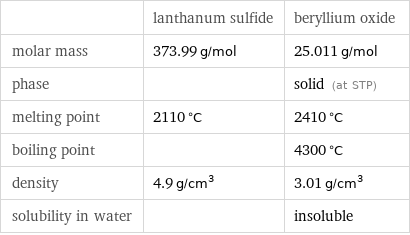
| lanthanum sulfide | beryllium oxide molar mass | 373.99 g/mol | 25.011 g/mol phase | | solid (at STP) melting point | 2110 °C | 2410 °C boiling point | | 4300 °C density | 4.9 g/cm^3 | 3.01 g/cm^3 solubility in water | | insoluble
Units
