Input interpretation
2, 2-bis(4-hydroxyphenyl)hexafluoropropane
Basic properties
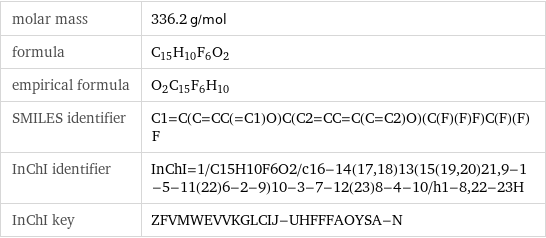
molar mass | 336.2 g/mol formula | C_15H_10F_6O_2 empirical formula | O_2C_15F_6H_10 SMILES identifier | C1=C(C=CC(=C1)O)C(C2=CC=C(C=C2)O)(C(F)(F)F)C(F)(F)F InChI identifier | InChI=1/C15H10F6O2/c16-14(17, 18)13(15(19, 20)21, 9-1-5-11(22)6-2-9)10-3-7-12(23)8-4-10/h1-8, 22-23H InChI key | ZFVMWEVVKGLCIJ-UHFFFAOYSA-N
Lewis structure
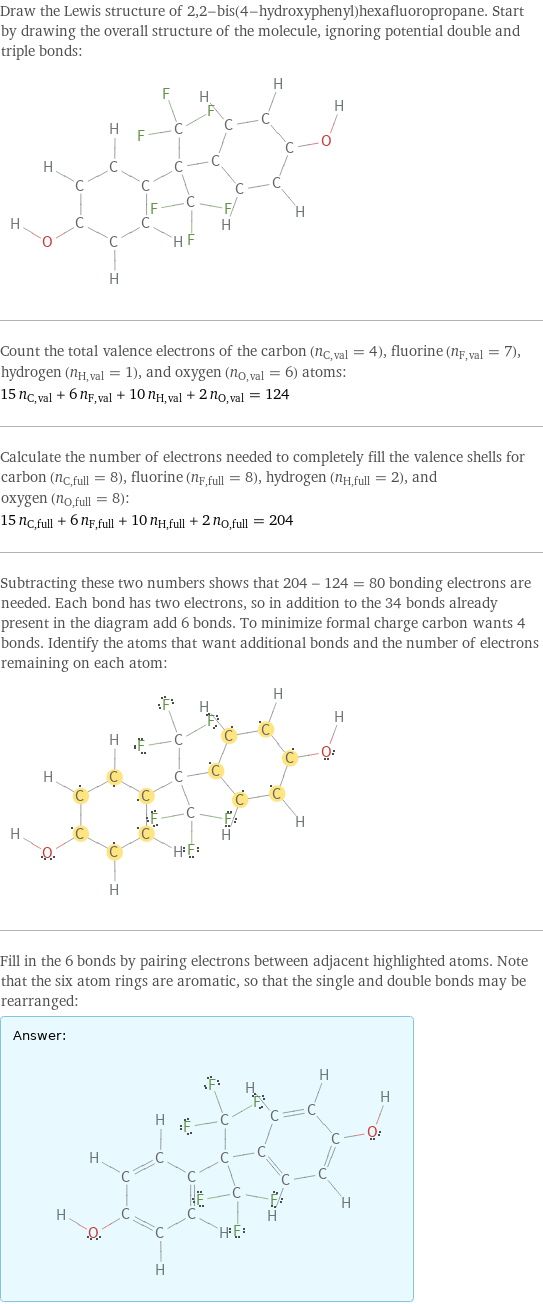
Draw the Lewis structure of 2, 2-bis(4-hydroxyphenyl)hexafluoropropane. Start by drawing the overall structure of the molecule, ignoring potential double and triple bonds: Count the total valence electrons of the carbon (n_C, val = 4), fluorine (n_F, val = 7), hydrogen (n_H, val = 1), and oxygen (n_O, val = 6) atoms: 15 n_C, val + 6 n_F, val + 10 n_H, val + 2 n_O, val = 124 Calculate the number of electrons needed to completely fill the valence shells for carbon (n_C, full = 8), fluorine (n_F, full = 8), hydrogen (n_H, full = 2), and oxygen (n_O, full = 8): 15 n_C, full + 6 n_F, full + 10 n_H, full + 2 n_O, full = 204 Subtracting these two numbers shows that 204 - 124 = 80 bonding electrons are needed. Each bond has two electrons, so in addition to the 34 bonds already present in the diagram add 6 bonds. To minimize formal charge carbon wants 4 bonds. Identify the atoms that want additional bonds and the number of electrons remaining on each atom: Fill in the 6 bonds by pairing electrons between adjacent highlighted atoms. Note that the six atom rings are aromatic, so that the single and double bonds may be rearranged: Answer: | |
Estimated thermodynamic properties
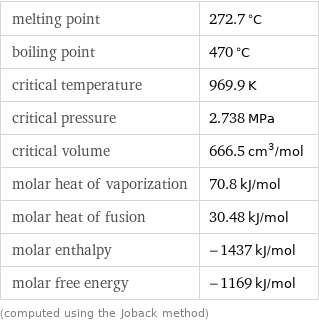
melting point | 272.7 °C boiling point | 470 °C critical temperature | 969.9 K critical pressure | 2.738 MPa critical volume | 666.5 cm^3/mol molar heat of vaporization | 70.8 kJ/mol molar heat of fusion | 30.48 kJ/mol molar enthalpy | -1437 kJ/mol molar free energy | -1169 kJ/mol (computed using the Joback method)
Units

Quantitative molecular descriptors
longest chain length | 11 atoms longest straight chain length | 5 atoms longest aliphatic chain length | 3 atoms aromatic atom count | 12 atoms H-bond acceptor count | 2 atoms H-bond donor count | 2 atoms
Elemental composition
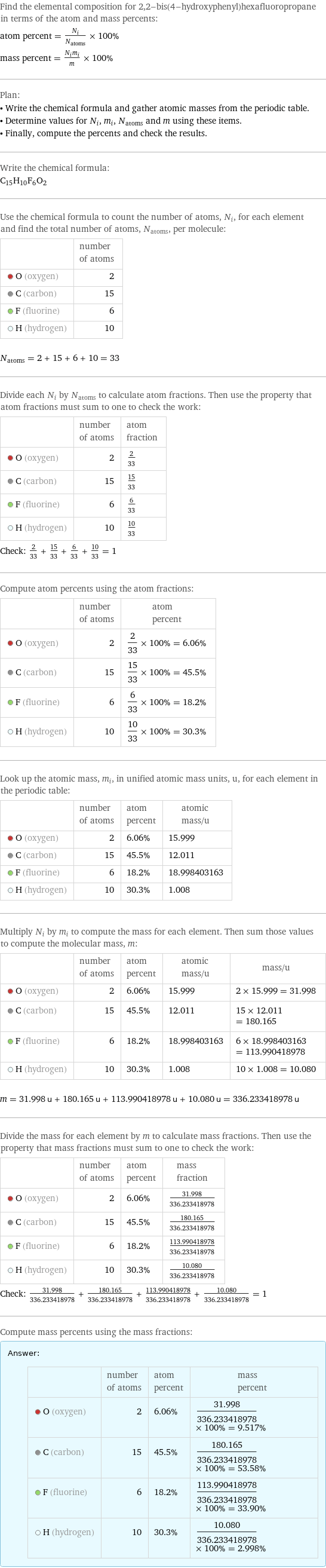
Find the elemental composition for 2, 2-bis(4-hydroxyphenyl)hexafluoropropane in terms of the atom and mass percents: atom percent = N_i/N_atoms × 100% mass percent = (N_im_i)/m × 100% Plan: • Write the chemical formula and gather atomic masses from the periodic table. • Determine values for N_i, m_i, N_atoms and m using these items. • Finally, compute the percents and check the results. Write the chemical formula: C_15H_10F_6O_2 Use the chemical formula to count the number of atoms, N_i, for each element and find the total number of atoms, N_atoms, per molecule: | number of atoms O (oxygen) | 2 C (carbon) | 15 F (fluorine) | 6 H (hydrogen) | 10 N_atoms = 2 + 15 + 6 + 10 = 33 Divide each N_i by N_atoms to calculate atom fractions. Then use the property that atom fractions must sum to one to check the work: | number of atoms | atom fraction O (oxygen) | 2 | 2/33 C (carbon) | 15 | 15/33 F (fluorine) | 6 | 6/33 H (hydrogen) | 10 | 10/33 Check: 2/33 + 15/33 + 6/33 + 10/33 = 1 Compute atom percents using the atom fractions: | number of atoms | atom percent O (oxygen) | 2 | 2/33 × 100% = 6.06% C (carbon) | 15 | 15/33 × 100% = 45.5% F (fluorine) | 6 | 6/33 × 100% = 18.2% H (hydrogen) | 10 | 10/33 × 100% = 30.3% Look up the atomic mass, m_i, in unified atomic mass units, u, for each element in the periodic table: | number of atoms | atom percent | atomic mass/u O (oxygen) | 2 | 6.06% | 15.999 C (carbon) | 15 | 45.5% | 12.011 F (fluorine) | 6 | 18.2% | 18.998403163 H (hydrogen) | 10 | 30.3% | 1.008 Multiply N_i by m_i to compute the mass for each element. Then sum those values to compute the molecular mass, m: | number of atoms | atom percent | atomic mass/u | mass/u O (oxygen) | 2 | 6.06% | 15.999 | 2 × 15.999 = 31.998 C (carbon) | 15 | 45.5% | 12.011 | 15 × 12.011 = 180.165 F (fluorine) | 6 | 18.2% | 18.998403163 | 6 × 18.998403163 = 113.990418978 H (hydrogen) | 10 | 30.3% | 1.008 | 10 × 1.008 = 10.080 m = 31.998 u + 180.165 u + 113.990418978 u + 10.080 u = 336.233418978 u Divide the mass for each element by m to calculate mass fractions. Then use the property that mass fractions must sum to one to check the work: | number of atoms | atom percent | mass fraction O (oxygen) | 2 | 6.06% | 31.998/336.233418978 C (carbon) | 15 | 45.5% | 180.165/336.233418978 F (fluorine) | 6 | 18.2% | 113.990418978/336.233418978 H (hydrogen) | 10 | 30.3% | 10.080/336.233418978 Check: 31.998/336.233418978 + 180.165/336.233418978 + 113.990418978/336.233418978 + 10.080/336.233418978 = 1 Compute mass percents using the mass fractions: Answer: | | | number of atoms | atom percent | mass percent O (oxygen) | 2 | 6.06% | 31.998/336.233418978 × 100% = 9.517% C (carbon) | 15 | 45.5% | 180.165/336.233418978 × 100% = 53.58% F (fluorine) | 6 | 18.2% | 113.990418978/336.233418978 × 100% = 33.90% H (hydrogen) | 10 | 30.3% | 10.080/336.233418978 × 100% = 2.998%
Elemental oxidation states
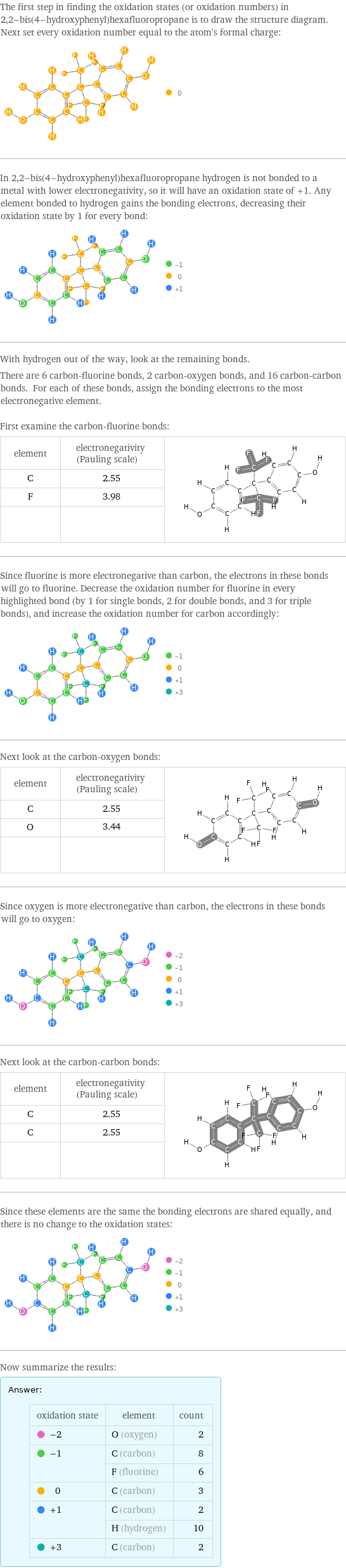
The first step in finding the oxidation states (or oxidation numbers) in 2, 2-bis(4-hydroxyphenyl)hexafluoropropane is to draw the structure diagram. Next set every oxidation number equal to the atom's formal charge: In 2, 2-bis(4-hydroxyphenyl)hexafluoropropane hydrogen is not bonded to a metal with lower electronegativity, so it will have an oxidation state of +1. Any element bonded to hydrogen gains the bonding electrons, decreasing their oxidation state by 1 for every bond: With hydrogen out of the way, look at the remaining bonds. There are 6 carbon-fluorine bonds, 2 carbon-oxygen bonds, and 16 carbon-carbon bonds. For each of these bonds, assign the bonding electrons to the most electronegative element. First examine the carbon-fluorine bonds: element | electronegativity (Pauling scale) | C | 2.55 | F | 3.98 | | | Since fluorine is more electronegative than carbon, the electrons in these bonds will go to fluorine. Decrease the oxidation number for fluorine in every highlighted bond (by 1 for single bonds, 2 for double bonds, and 3 for triple bonds), and increase the oxidation number for carbon accordingly: Next look at the carbon-oxygen bonds: element | electronegativity (Pauling scale) | C | 2.55 | O | 3.44 | | | Since oxygen is more electronegative than carbon, the electrons in these bonds will go to oxygen: Next look at the carbon-carbon bonds: element | electronegativity (Pauling scale) | C | 2.55 | C | 2.55 | | | Since these elements are the same the bonding electrons are shared equally, and there is no change to the oxidation states: Now summarize the results: Answer: | | oxidation state | element | count -2 | O (oxygen) | 2 -1 | C (carbon) | 8 | F (fluorine) | 6 0 | C (carbon) | 3 +1 | C (carbon) | 2 | H (hydrogen) | 10 +3 | C (carbon) | 2
Orbital hybridization
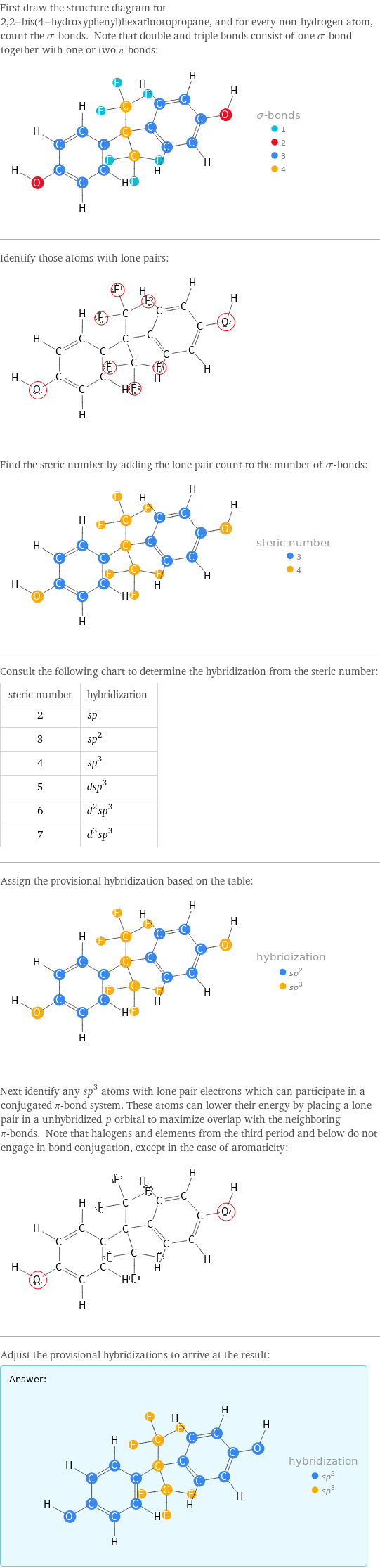
First draw the structure diagram for 2, 2-bis(4-hydroxyphenyl)hexafluoropropane, and for every non-hydrogen atom, count the σ-bonds. Note that double and triple bonds consist of one σ-bond together with one or two π-bonds: Identify those atoms with lone pairs: Find the steric number by adding the lone pair count to the number of σ-bonds: Consult the following chart to determine the hybridization from the steric number: steric number | hybridization 2 | sp 3 | sp^2 4 | sp^3 5 | dsp^3 6 | d^2sp^3 7 | d^3sp^3 Assign the provisional hybridization based on the table: Next identify any sp^3 atoms with lone pair electrons which can participate in a conjugated π-bond system. These atoms can lower their energy by placing a lone pair in a unhybridized p orbital to maximize overlap with the neighboring π-bonds. Note that halogens and elements from the third period and below do not engage in bond conjugation, except in the case of aromaticity: Adjust the provisional hybridizations to arrive at the result: Answer: | |
Topological indices
vertex count | 33 edge count | 34 Schultz index | 9638 Wiener index | 2464 Hosoya index | 1.845×10^6 Balaban index | 2.87