Input interpretation
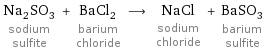
Na_2SO_3 sodium sulfite + BaCl_2 barium chloride ⟶ NaCl sodium chloride + BaSO_3 barium sulfite
Chemical names and formulas
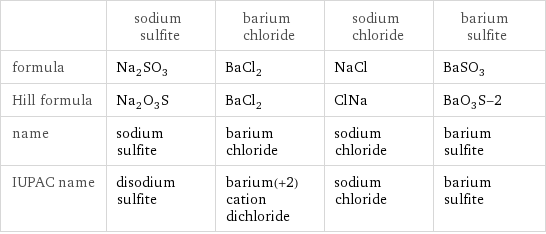
| sodium sulfite | barium chloride | sodium chloride | barium sulfite formula | Na_2SO_3 | BaCl_2 | NaCl | BaSO_3 Hill formula | Na_2O_3S | BaCl_2 | ClNa | BaO_3S-2 name | sodium sulfite | barium chloride | sodium chloride | barium sulfite IUPAC name | disodium sulfite | barium(+2) cation dichloride | sodium chloride | barium sulfite
Substance properties
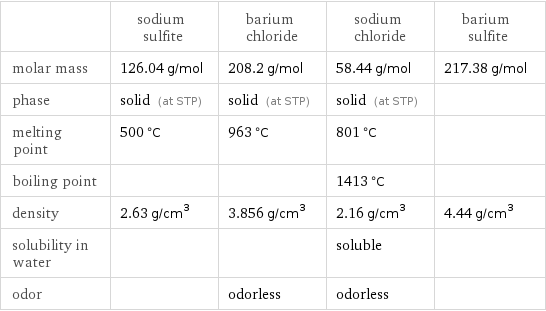
| sodium sulfite | barium chloride | sodium chloride | barium sulfite molar mass | 126.04 g/mol | 208.2 g/mol | 58.44 g/mol | 217.38 g/mol phase | solid (at STP) | solid (at STP) | solid (at STP) | melting point | 500 °C | 963 °C | 801 °C | boiling point | | | 1413 °C | density | 2.63 g/cm^3 | 3.856 g/cm^3 | 2.16 g/cm^3 | 4.44 g/cm^3 solubility in water | | | soluble | odor | | odorless | odorless |
Units
