Input interpretation
air
Chemical names and formulas
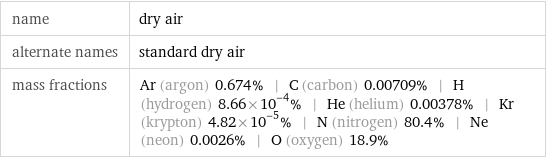
name | dry air alternate names | standard dry air mass fractions | Ar (argon) 0.674% | C (carbon) 0.00709% | H (hydrogen) 8.66×10^-4% | He (helium) 0.00378% | Kr (krypton) 4.82×10^-5% | N (nitrogen) 80.4% | Ne (neon) 0.0026% | O (oxygen) 18.9%
Basic properties
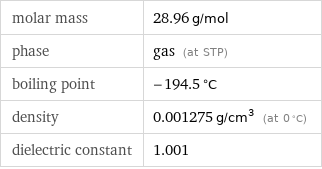
molar mass | 28.96 g/mol phase | gas (at STP) boiling point | -194.5 °C density | 0.001275 g/cm^3 (at 0 °C) dielectric constant | 1.001
Gas properties (at STP)
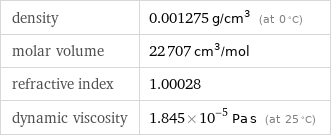
density | 0.001275 g/cm^3 (at 0 °C) molar volume | 22707 cm^3/mol refractive index | 1.00028 dynamic viscosity | 1.845×10^-5 Pa s (at 25 °C)
Units
Thermodynamic properties
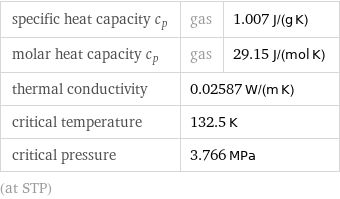
specific heat capacity c_p | gas | 1.007 J/(g K) molar heat capacity c_p | gas | 29.15 J/(mol K) thermal conductivity | 0.02587 W/(m K) | critical temperature | 132.5 K | critical pressure | 3.766 MPa | (at STP)
Phase diagram
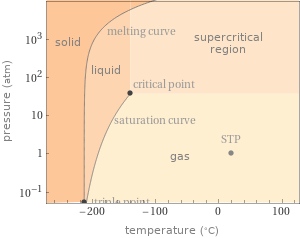
Phase diagram
Units
