Input interpretation
potassium bisulfate | lithium hydroxide monohydrate
Chemical names and formulas
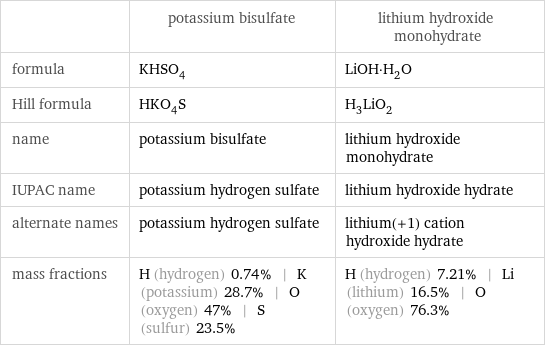
| potassium bisulfate | lithium hydroxide monohydrate formula | KHSO_4 | LiOH·H_2O Hill formula | HKO_4S | H_3LiO_2 name | potassium bisulfate | lithium hydroxide monohydrate IUPAC name | potassium hydrogen sulfate | lithium hydroxide hydrate alternate names | potassium hydrogen sulfate | lithium(+1) cation hydroxide hydrate mass fractions | H (hydrogen) 0.74% | K (potassium) 28.7% | O (oxygen) 47% | S (sulfur) 23.5% | H (hydrogen) 7.21% | Li (lithium) 16.5% | O (oxygen) 76.3%
Structure diagrams
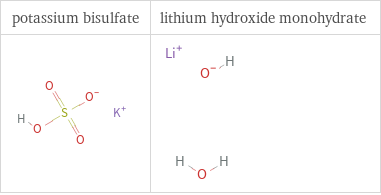
Structure diagrams
Basic properties
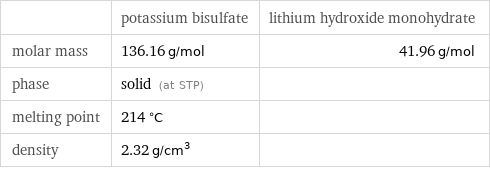
| potassium bisulfate | lithium hydroxide monohydrate molar mass | 136.16 g/mol | 41.96 g/mol phase | solid (at STP) | melting point | 214 °C | density | 2.32 g/cm^3 |
Units

Solid properties

| potassium bisulfate density | 2.32 g/cm^3
Units
