Input interpretation
ammonium chloride
Chemical names and formulas
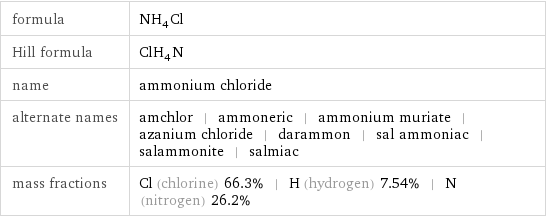
formula | NH_4Cl Hill formula | ClH_4N name | ammonium chloride alternate names | amchlor | ammoneric | ammonium muriate | azanium chloride | darammon | sal ammoniac | salammonite | salmiac mass fractions | Cl (chlorine) 66.3% | H (hydrogen) 7.54% | N (nitrogen) 26.2%
Structure diagram

Structure diagram
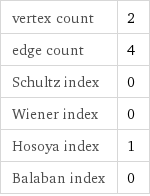
vertex count | 2 edge count | 4 Schultz index | 0 Wiener index | 0 Hosoya index | 1 Balaban index | 0
Basic properties
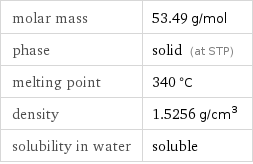
molar mass | 53.49 g/mol phase | solid (at STP) melting point | 340 °C density | 1.5256 g/cm^3 solubility in water | soluble
Units

Solid properties (at STP)
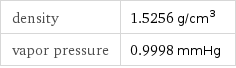
density | 1.5256 g/cm^3 vapor pressure | 0.9998 mmHg
Units

Thermodynamic properties
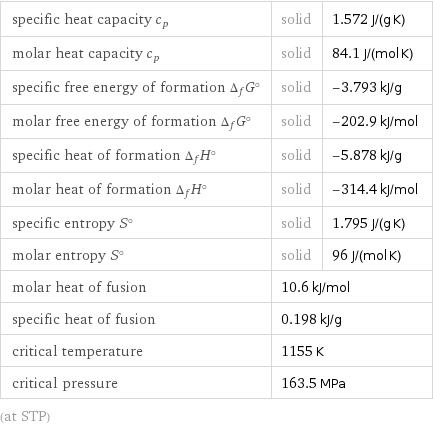
specific heat capacity c_p | solid | 1.572 J/(g K) molar heat capacity c_p | solid | 84.1 J/(mol K) specific free energy of formation Δ_fG° | solid | -3.793 kJ/g molar free energy of formation Δ_fG° | solid | -202.9 kJ/mol specific heat of formation Δ_fH° | solid | -5.878 kJ/g molar heat of formation Δ_fH° | solid | -314.4 kJ/mol specific entropy S° | solid | 1.795 J/(g K) molar entropy S° | solid | 96 J/(mol K) molar heat of fusion | 10.6 kJ/mol | specific heat of fusion | 0.198 kJ/g | critical temperature | 1155 K | critical pressure | 163.5 MPa | (at STP)
Chemical identifiers
![CAS number | 12125-02-9 Beilstein number | 4371014 PubChem CID number | 25517 PubChem SID number | 24855359 SMILES identifier | [NH4+].[Cl-]](../image_source/198ba3f7370af5f347e54d9ceaf62a42.png)
CAS number | 12125-02-9 Beilstein number | 4371014 PubChem CID number | 25517 PubChem SID number | 24855359 SMILES identifier | [NH4+].[Cl-]
NFPA label

NFPA label
Safety properties

autoignition point | 400 °C
Toxicity properties

RTECS classes | drug | mutagen | human data | primary irritant
Ion equivalents

(NH_4)^+ (ammonium cation) | 1 Cl^- (chloride anion) | 1