Input interpretation
arsenic trioxide | lead(II) telluride
Chemical names and formulas
![| arsenic trioxide | lead(II) telluride formula | As_2O_3 | PbTe Hill formula | As_2O_3 | PbTe name | arsenic trioxide | lead(II) telluride IUPAC name | 2, 4, 5-trioxa-1, 3-diarsabicyclo[1.1.1]pentane | telluroxolead alternate names | arsenic(III) oxide | tellanylidenelead | telluroxolead mass fractions | As (arsenic) 75.7% | O (oxygen) 24.3% | Pb (lead) 61.9% | Te (tellurium) 38.1%](../image_source/aacbb9ecac09209d22a1f5e73419581b.png)
| arsenic trioxide | lead(II) telluride formula | As_2O_3 | PbTe Hill formula | As_2O_3 | PbTe name | arsenic trioxide | lead(II) telluride IUPAC name | 2, 4, 5-trioxa-1, 3-diarsabicyclo[1.1.1]pentane | telluroxolead alternate names | arsenic(III) oxide | tellanylidenelead | telluroxolead mass fractions | As (arsenic) 75.7% | O (oxygen) 24.3% | Pb (lead) 61.9% | Te (tellurium) 38.1%
Structure diagrams
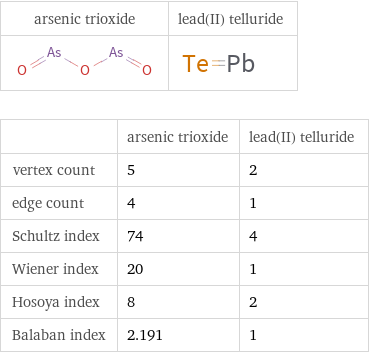
| arsenic trioxide | lead(II) telluride vertex count | 5 | 2 edge count | 4 | 1 Schultz index | 74 | 4 Wiener index | 20 | 1 Hosoya index | 8 | 2 Balaban index | 2.191 | 1
Basic properties
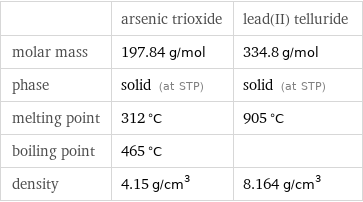
| arsenic trioxide | lead(II) telluride molar mass | 197.84 g/mol | 334.8 g/mol phase | solid (at STP) | solid (at STP) melting point | 312 °C | 905 °C boiling point | 465 °C | density | 4.15 g/cm^3 | 8.164 g/cm^3
Units

Thermodynamic properties
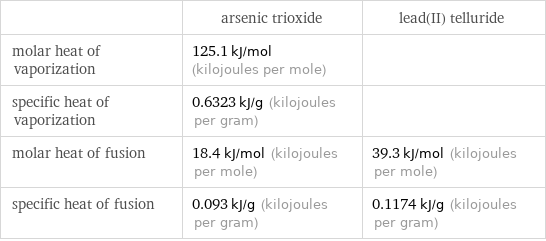
| arsenic trioxide | lead(II) telluride molar heat of vaporization | 125.1 kJ/mol (kilojoules per mole) | specific heat of vaporization | 0.6323 kJ/g (kilojoules per gram) | molar heat of fusion | 18.4 kJ/mol (kilojoules per mole) | 39.3 kJ/mol (kilojoules per mole) specific heat of fusion | 0.093 kJ/g (kilojoules per gram) | 0.1174 kJ/g (kilojoules per gram)