Input interpretation

organic solvents | freezing point
Summary
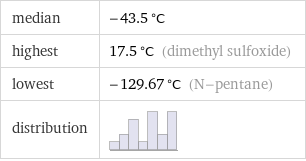
median | -43.5 °C highest | 17.5 °C (dimethyl sulfoxide) lowest | -129.67 °C (N-pentane) distribution |
Distribution plots
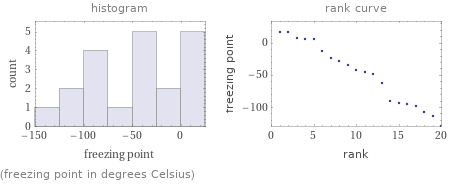
(freezing point in degrees Celsius)
Freezing point rankings
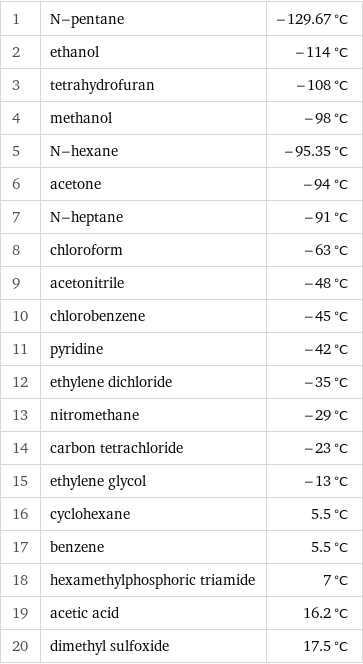
1 | N-pentane | -129.67 °C 2 | ethanol | -114 °C 3 | tetrahydrofuran | -108 °C 4 | methanol | -98 °C 5 | N-hexane | -95.35 °C 6 | acetone | -94 °C 7 | N-heptane | -91 °C 8 | chloroform | -63 °C 9 | acetonitrile | -48 °C 10 | chlorobenzene | -45 °C 11 | pyridine | -42 °C 12 | ethylene dichloride | -35 °C 13 | nitromethane | -29 °C 14 | carbon tetrachloride | -23 °C 15 | ethylene glycol | -13 °C 16 | cyclohexane | 5.5 °C 17 | benzene | 5.5 °C 18 | hexamethylphosphoric triamide | 7 °C 19 | acetic acid | 16.2 °C 20 | dimethyl sulfoxide | 17.5 °C
Unit conversions for median freezing point -43.5 °C
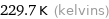
229.7 K (kelvins)

-46.3 °F (degrees Fahrenheit)
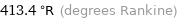
413.4 °R (degrees Rankine)
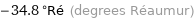
-34.8 °Ré (degrees Réaumur)

-15.34 °Rø (degrees Rømer)
Comparison for median freezing point -43.5 °C

25.72 °C below temperature of the ice/salt mixture defining the zero point of the Fahrenheit temperature scale (0 °F)
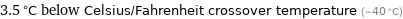
3.5 °C below Celsius/Fahrenheit crossover temperature (-40 °C)
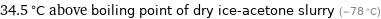
34.5 °C above boiling point of dry ice-acetone slurry (-78 °C)
Corresponding quantities

Thermodynamic energy E from E = kT: | 20 meV (millielectronvolts)
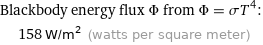
Blackbody energy flux Φ from Φ = σT^4: | 158 W/m^2 (watts per square meter)

Approximate luminous exitance from a planar blackbody radiator perpendicular to its surface: | 4.8×10^-30 lx (lux)