Input interpretation
carbonate anion | bromide anion | iodide anion
Structure diagrams
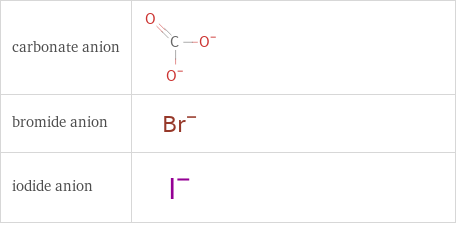
Structure diagrams
General properties
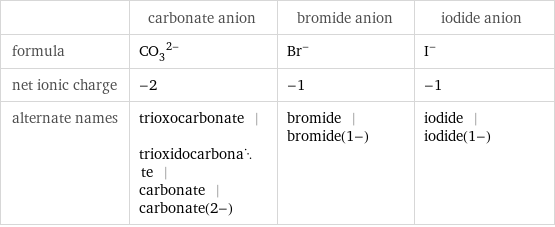
| carbonate anion | bromide anion | iodide anion formula | (CO_3)^(2-) | Br^- | I^- net ionic charge | -2 | -1 | -1 alternate names | trioxocarbonate | trioxidocarbonate | carbonate | carbonate(2-) | bromide | bromide(1-) | iodide | iodide(1-)
Ionic radii

| carbonate anion | bromide anion | iodide anion 6-coordinate ionic radius | | 196 pm | 220 pm thermochemical radius | 178 pm | |
Units

Other properties
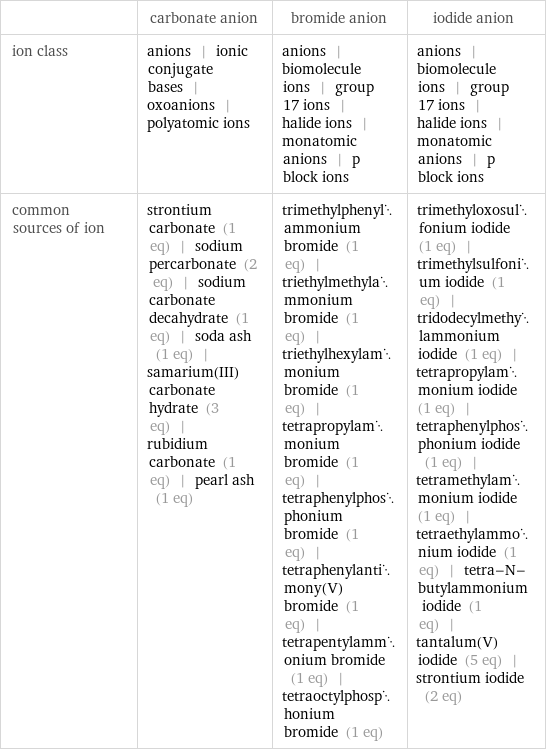
| carbonate anion | bromide anion | iodide anion ion class | anions | ionic conjugate bases | oxoanions | polyatomic ions | anions | biomolecule ions | group 17 ions | halide ions | monatomic anions | p block ions | anions | biomolecule ions | group 17 ions | halide ions | monatomic anions | p block ions common sources of ion | strontium carbonate (1 eq) | sodium percarbonate (2 eq) | sodium carbonate decahydrate (1 eq) | soda ash (1 eq) | samarium(III) carbonate hydrate (3 eq) | rubidium carbonate (1 eq) | pearl ash (1 eq) | trimethylphenylammonium bromide (1 eq) | triethylmethylammonium bromide (1 eq) | triethylhexylammonium bromide (1 eq) | tetrapropylammonium bromide (1 eq) | tetraphenylphosphonium bromide (1 eq) | tetraphenylantimony(V) bromide (1 eq) | tetrapentylammonium bromide (1 eq) | tetraoctylphosphonium bromide (1 eq) | trimethyloxosulfonium iodide (1 eq) | trimethylsulfonium iodide (1 eq) | tridodecylmethylammonium iodide (1 eq) | tetrapropylammonium iodide (1 eq) | tetraphenylphosphonium iodide (1 eq) | tetramethylammonium iodide (1 eq) | tetraethylammonium iodide (1 eq) | tetra-N-butylammonium iodide (1 eq) | tantalum(V) iodide (5 eq) | strontium iodide (2 eq)