Input interpretation

colorants | molar mass
Summary
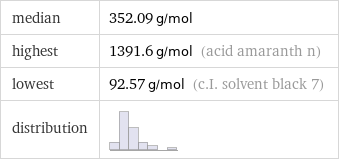
median | 352.09 g/mol highest | 1391.6 g/mol (acid amaranth n) lowest | 92.57 g/mol (c.I. solvent black 7) distribution |
Units

Distribution plots
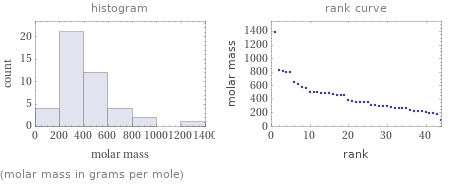
(molar mass in grams per mole)
Molar mass rankings
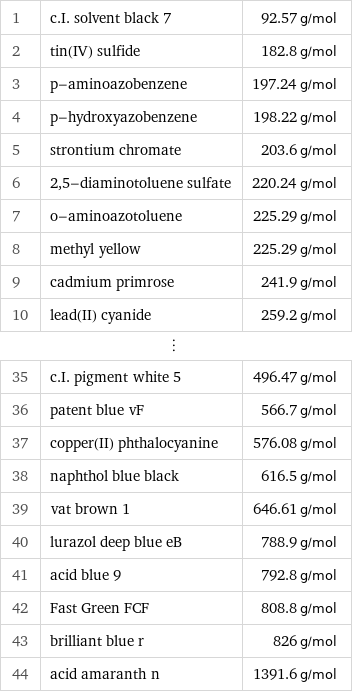
1 | c.I. solvent black 7 | 92.57 g/mol 2 | tin(IV) sulfide | 182.8 g/mol 3 | p-aminoazobenzene | 197.24 g/mol 4 | p-hydroxyazobenzene | 198.22 g/mol 5 | strontium chromate | 203.6 g/mol 6 | 2, 5-diaminotoluene sulfate | 220.24 g/mol 7 | o-aminoazotoluene | 225.29 g/mol 8 | methyl yellow | 225.29 g/mol 9 | cadmium primrose | 241.9 g/mol 10 | lead(II) cyanide | 259.2 g/mol ⋮ | | 35 | c.I. pigment white 5 | 496.47 g/mol 36 | patent blue vF | 566.7 g/mol 37 | copper(II) phthalocyanine | 576.08 g/mol 38 | naphthol blue black | 616.5 g/mol 39 | vat brown 1 | 646.61 g/mol 40 | lurazol deep blue eB | 788.9 g/mol 41 | acid blue 9 | 792.8 g/mol 42 | Fast Green FCF | 808.8 g/mol 43 | brilliant blue r | 826 g/mol 44 | acid amaranth n | 1391.6 g/mol
Unit conversion for median molar mass 352.09 g/mol
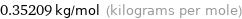
0.35209 kg/mol (kilograms per mole)
Comparisons for median molar mass 352.09 g/mol
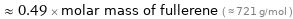
≈ 0.49 × molar mass of fullerene ( ≈ 721 g/mol )
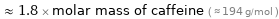
≈ 1.8 × molar mass of caffeine ( ≈ 194 g/mol )
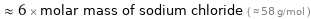
≈ 6 × molar mass of sodium chloride ( ≈ 58 g/mol )
Corresponding quantities
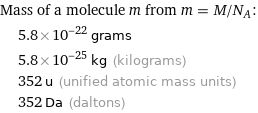
Mass of a molecule m from m = M/N_A: | 5.8×10^-22 grams | 5.8×10^-25 kg (kilograms) | 352 u (unified atomic mass units) | 352 Da (daltons)
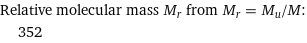
Relative molecular mass M_r from M_r = M_u/M: | 352