Input interpretation
carbon (chemical element) | hydrogen (chemical element) | vanadium (chemical element)
Periodic table location
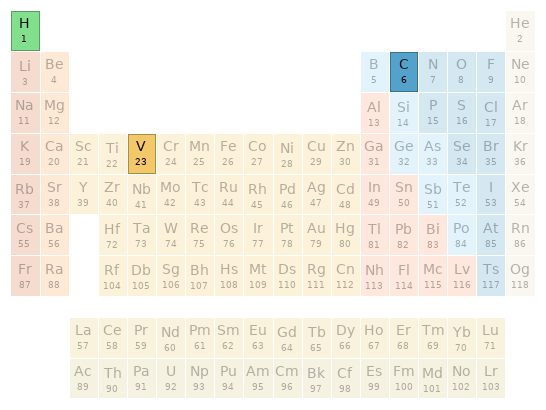
Periodic table location
Images
Images
Basic elemental properties
![| carbon | hydrogen | vanadium atomic symbol | C | H | V atomic number | 6 | 1 | 23 short electronic configuration | [He]2s^22p^2 | 1s^1 | [Ar]4s^23d^3 Aufbau diagram | 2p 2s | 1s | 3d 4s block | p | s | d group | 14 | 1 | 5 period | 2 | 1 | 4 atomic mass | 12.011 u | 1.008 u | 50.9415 u half-life | (stable) | (stable) | (stable)](../image_source/0fd95b2cb059a5bbc01a62ef442f053f.png)
| carbon | hydrogen | vanadium atomic symbol | C | H | V atomic number | 6 | 1 | 23 short electronic configuration | [He]2s^22p^2 | 1s^1 | [Ar]4s^23d^3 Aufbau diagram | 2p 2s | 1s | 3d 4s block | p | s | d group | 14 | 1 | 5 period | 2 | 1 | 4 atomic mass | 12.011 u | 1.008 u | 50.9415 u half-life | (stable) | (stable) | (stable)
Thermodynamic properties
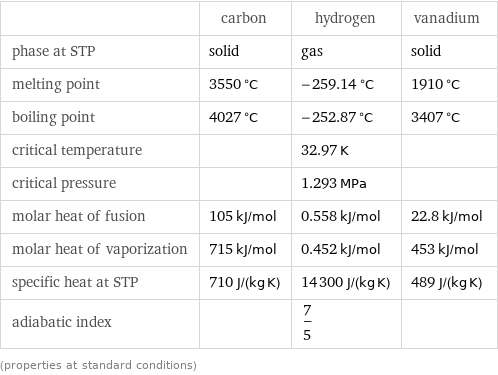
| carbon | hydrogen | vanadium phase at STP | solid | gas | solid melting point | 3550 °C | -259.14 °C | 1910 °C boiling point | 4027 °C | -252.87 °C | 3407 °C critical temperature | | 32.97 K | critical pressure | | 1.293 MPa | molar heat of fusion | 105 kJ/mol | 0.558 kJ/mol | 22.8 kJ/mol molar heat of vaporization | 715 kJ/mol | 0.452 kJ/mol | 453 kJ/mol specific heat at STP | 710 J/(kg K) | 14300 J/(kg K) | 489 J/(kg K) adiabatic index | | 7/5 | (properties at standard conditions)
Material properties
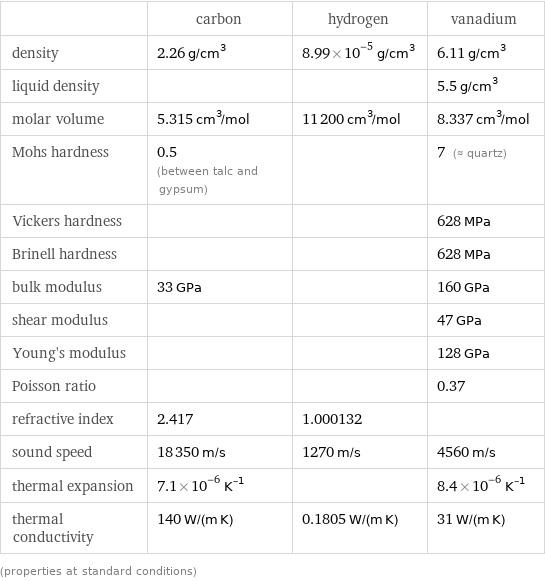
| carbon | hydrogen | vanadium density | 2.26 g/cm^3 | 8.99×10^-5 g/cm^3 | 6.11 g/cm^3 liquid density | | | 5.5 g/cm^3 molar volume | 5.315 cm^3/mol | 11200 cm^3/mol | 8.337 cm^3/mol Mohs hardness | 0.5 (between talc and gypsum) | | 7 (≈ quartz) Vickers hardness | | | 628 MPa Brinell hardness | | | 628 MPa bulk modulus | 33 GPa | | 160 GPa shear modulus | | | 47 GPa Young's modulus | | | 128 GPa Poisson ratio | | | 0.37 refractive index | 2.417 | 1.000132 | sound speed | 18350 m/s | 1270 m/s | 4560 m/s thermal expansion | 7.1×10^-6 K^(-1) | | 8.4×10^-6 K^(-1) thermal conductivity | 140 W/(m K) | 0.1805 W/(m K) | 31 W/(m K) (properties at standard conditions)
Electromagnetic properties
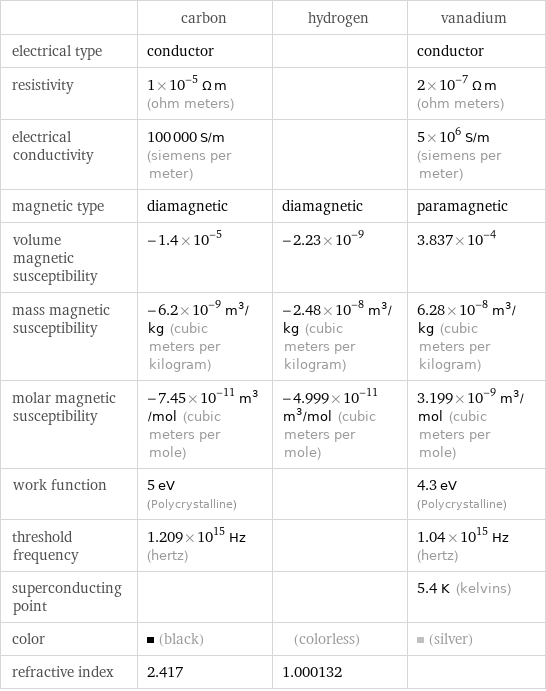
| carbon | hydrogen | vanadium electrical type | conductor | | conductor resistivity | 1×10^-5 Ω m (ohm meters) | | 2×10^-7 Ω m (ohm meters) electrical conductivity | 100000 S/m (siemens per meter) | | 5×10^6 S/m (siemens per meter) magnetic type | diamagnetic | diamagnetic | paramagnetic volume magnetic susceptibility | -1.4×10^-5 | -2.23×10^-9 | 3.837×10^-4 mass magnetic susceptibility | -6.2×10^-9 m^3/kg (cubic meters per kilogram) | -2.48×10^-8 m^3/kg (cubic meters per kilogram) | 6.28×10^-8 m^3/kg (cubic meters per kilogram) molar magnetic susceptibility | -7.45×10^-11 m^3/mol (cubic meters per mole) | -4.999×10^-11 m^3/mol (cubic meters per mole) | 3.199×10^-9 m^3/mol (cubic meters per mole) work function | 5 eV (Polycrystalline) | | 4.3 eV (Polycrystalline) threshold frequency | 1.209×10^15 Hz (hertz) | | 1.04×10^15 Hz (hertz) superconducting point | | | 5.4 K (kelvins) color | (black) | (colorless) | (silver) refractive index | 2.417 | 1.000132 |
Reactivity
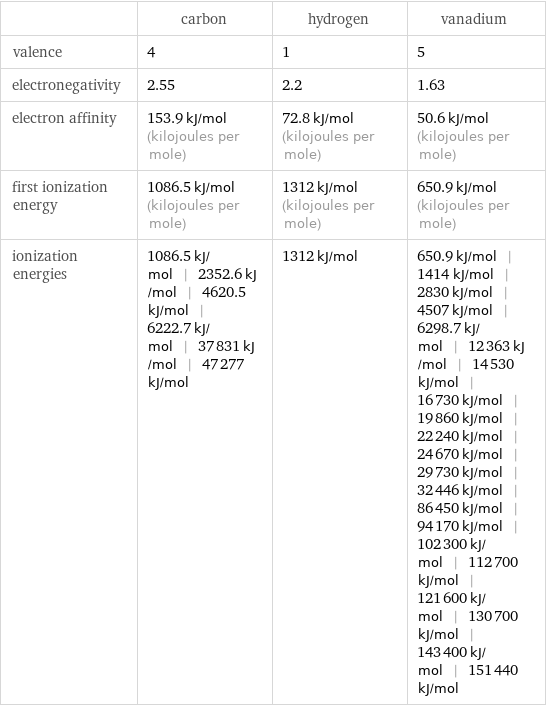
| carbon | hydrogen | vanadium valence | 4 | 1 | 5 electronegativity | 2.55 | 2.2 | 1.63 electron affinity | 153.9 kJ/mol (kilojoules per mole) | 72.8 kJ/mol (kilojoules per mole) | 50.6 kJ/mol (kilojoules per mole) first ionization energy | 1086.5 kJ/mol (kilojoules per mole) | 1312 kJ/mol (kilojoules per mole) | 650.9 kJ/mol (kilojoules per mole) ionization energies | 1086.5 kJ/mol | 2352.6 kJ/mol | 4620.5 kJ/mol | 6222.7 kJ/mol | 37831 kJ/mol | 47277 kJ/mol | 1312 kJ/mol | 650.9 kJ/mol | 1414 kJ/mol | 2830 kJ/mol | 4507 kJ/mol | 6298.7 kJ/mol | 12363 kJ/mol | 14530 kJ/mol | 16730 kJ/mol | 19860 kJ/mol | 22240 kJ/mol | 24670 kJ/mol | 29730 kJ/mol | 32446 kJ/mol | 86450 kJ/mol | 94170 kJ/mol | 102300 kJ/mol | 112700 kJ/mol | 121600 kJ/mol | 130700 kJ/mol | 143400 kJ/mol | 151440 kJ/mol
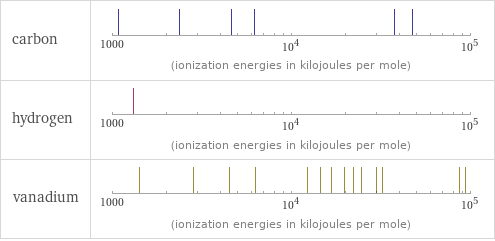
Reactivity
Atomic properties
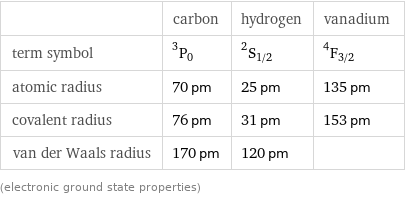
| carbon | hydrogen | vanadium term symbol | ^3P_0 | ^2S_(1/2) | ^4F_(3/2) atomic radius | 70 pm | 25 pm | 135 pm covalent radius | 76 pm | 31 pm | 153 pm van der Waals radius | 170 pm | 120 pm | (electronic ground state properties)
Abundances
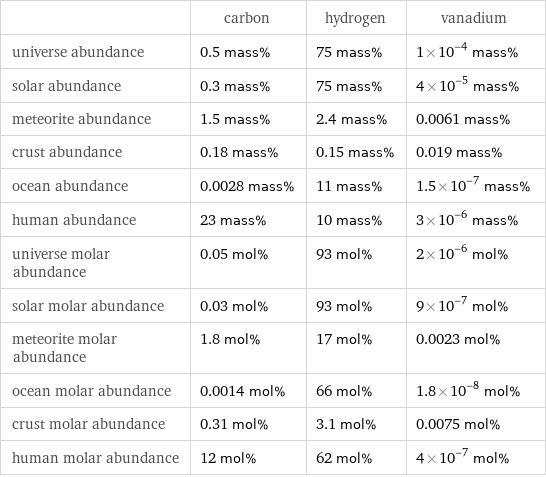
| carbon | hydrogen | vanadium universe abundance | 0.5 mass% | 75 mass% | 1×10^-4 mass% solar abundance | 0.3 mass% | 75 mass% | 4×10^-5 mass% meteorite abundance | 1.5 mass% | 2.4 mass% | 0.0061 mass% crust abundance | 0.18 mass% | 0.15 mass% | 0.019 mass% ocean abundance | 0.0028 mass% | 11 mass% | 1.5×10^-7 mass% human abundance | 23 mass% | 10 mass% | 3×10^-6 mass% universe molar abundance | 0.05 mol% | 93 mol% | 2×10^-6 mol% solar molar abundance | 0.03 mol% | 93 mol% | 9×10^-7 mol% meteorite molar abundance | 1.8 mol% | 17 mol% | 0.0023 mol% ocean molar abundance | 0.0014 mol% | 66 mol% | 1.8×10^-8 mol% crust molar abundance | 0.31 mol% | 3.1 mol% | 0.0075 mol% human molar abundance | 12 mol% | 62 mol% | 4×10^-7 mol%
Nuclear properties
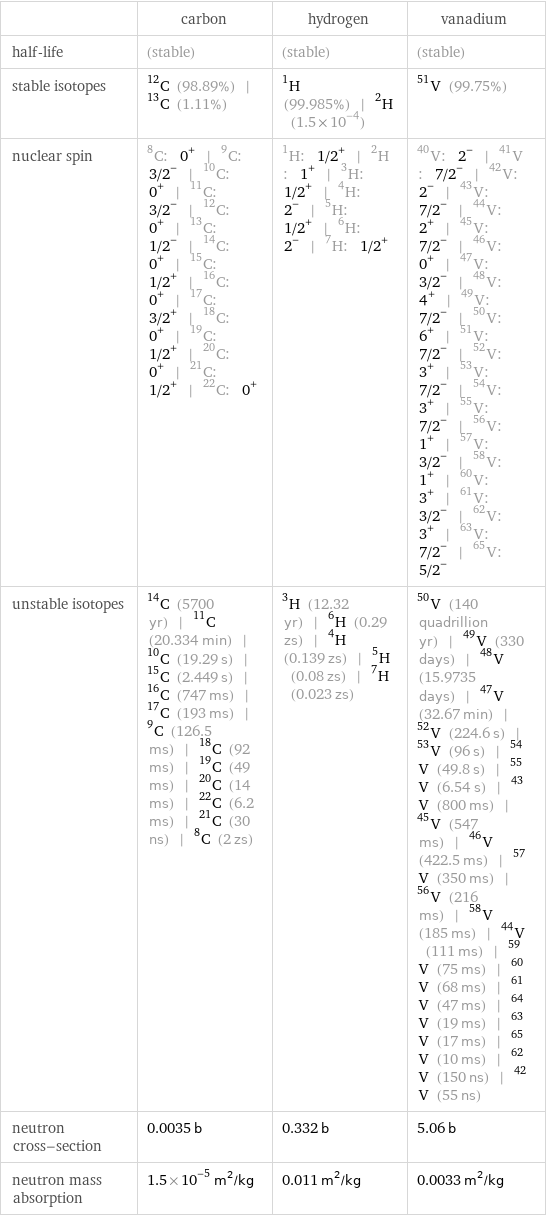
| carbon | hydrogen | vanadium half-life | (stable) | (stable) | (stable) stable isotopes | C-12 (98.89%) | C-13 (1.11%) | H-1 (99.985%) | H-2 (1.5×10^-4) | V-51 (99.75%) nuclear spin | C-8: 0^+ | C-9: 3/2^- | C-10: 0^+ | C-11: 3/2^- | C-12: 0^+ | C-13: 1/2^- | C-14: 0^+ | C-15: 1/2^+ | C-16: 0^+ | C-17: 3/2^+ | C-18: 0^+ | C-19: 1/2^+ | C-20: 0^+ | C-21: 1/2^+ | C-22: 0^+ | H-1: 1/2^+ | H-2: 1^+ | H-3: 1/2^+ | H-4: 2^- | H-5: 1/2^+ | H-6: 2^- | H-7: 1/2^+ | V-40: 2^- | V-41: 7/2^- | V-42: 2^- | V-43: 7/2^- | V-44: 2^+ | V-45: 7/2^- | V-46: 0^+ | V-47: 3/2^- | V-48: 4^+ | V-49: 7/2^- | V-50: 6^+ | V-51: 7/2^- | V-52: 3^+ | V-53: 7/2^- | V-54: 3^+ | V-55: 7/2^- | V-56: 1^+ | V-57: 3/2^- | V-58: 1^+ | V-60: 3^+ | V-61: 3/2^- | V-62: 3^+ | V-63: 7/2^- | V-65: 5/2^- unstable isotopes | C-14 (5700 yr) | C-11 (20.334 min) | C-10 (19.29 s) | C-15 (2.449 s) | C-16 (747 ms) | C-17 (193 ms) | C-9 (126.5 ms) | C-18 (92 ms) | C-19 (49 ms) | C-20 (14 ms) | C-22 (6.2 ms) | C-21 (30 ns) | C-8 (2 zs) | H-3 (12.32 yr) | H-6 (0.29 zs) | H-4 (0.139 zs) | H-5 (0.08 zs) | H-7 (0.023 zs) | V-50 (140 quadrillion yr) | V-49 (330 days) | V-48 (15.9735 days) | V-47 (32.67 min) | V-52 (224.6 s) | V-53 (96 s) | V-54 (49.8 s) | V-55 (6.54 s) | V-43 (800 ms) | V-45 (547 ms) | V-46 (422.5 ms) | V-57 (350 ms) | V-56 (216 ms) | V-58 (185 ms) | V-44 (111 ms) | V-59 (75 ms) | V-60 (68 ms) | V-61 (47 ms) | V-64 (19 ms) | V-63 (17 ms) | V-65 (10 ms) | V-62 (150 ns) | V-42 (55 ns) neutron cross-section | 0.0035 b | 0.332 b | 5.06 b neutron mass absorption | 1.5×10^-5 m^2/kg | 0.011 m^2/kg | 0.0033 m^2/kg
Identifiers
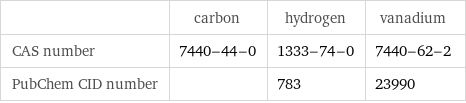
| carbon | hydrogen | vanadium CAS number | 7440-44-0 | 1333-74-0 | 7440-62-2 PubChem CID number | | 783 | 23990