Input interpretation
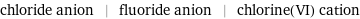
chloride anion | fluoride anion | chlorine(VI) cation
Structure diagrams
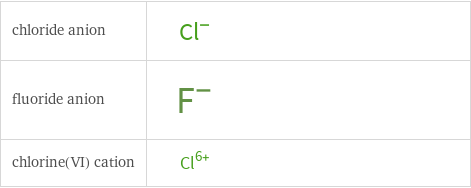
Structure diagrams
General properties
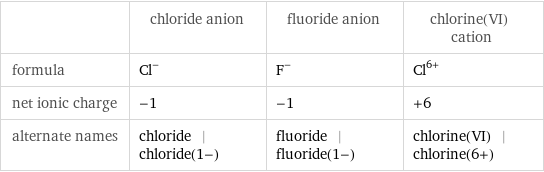
| chloride anion | fluoride anion | chlorine(VI) cation formula | Cl^- | F^- | Cl^(6+) net ionic charge | -1 | -1 | +6 alternate names | chloride | chloride(1-) | fluoride | fluoride(1-) | chlorine(VI) | chlorine(6+)
Ionic radii

| chloride anion | fluoride anion 6-coordinate ionic radius | 184 pm | 133 pm
Units

Other properties

| chloride anion | fluoride anion | chlorine(VI) cation ion class | anions | biomolecule ions | group 17 ions | halide ions | monatomic anions | p block ions | anions | biomolecule ions | group 17 ions | halide ions | monatomic anions | p block ions | ionic weak bases | cations | group 17 ions | p block ions common sources of ion | zinc chloride (2 eq) | trimethylsulfoxonium chloride (1 eq) | triethylmethylammonium chloride (1 eq) | tetrapropylammonium chloride (1 eq) | tetrapentylammonium chloride (1 eq) | tetramethylphosphonium chloride (1 eq) | tetramethylammonium chloride (1 eq) | tetrahexylammonium chloride (1 eq) | tetraheptylammonium chloride (1 eq) | vanadium(IV) fluoride (5 eq) | tropylium tetrafluoroborate (1 eq) | tetramethylammonium fluoride tetrahydrate (1 eq) | strontium fluoride (2 eq) | sodium hydrogen fluoride (1 eq) | sodium hexafluorozirconate (6 eq) | sodium hexafluorotitanate (2 eq) | sodium hexafluoroarsenate(V) (6 eq) | sodium hexafluoroantimonate (6 eq) | sodium fluoride (1 eq) |