Input interpretation
tropylium cation
Lewis structure
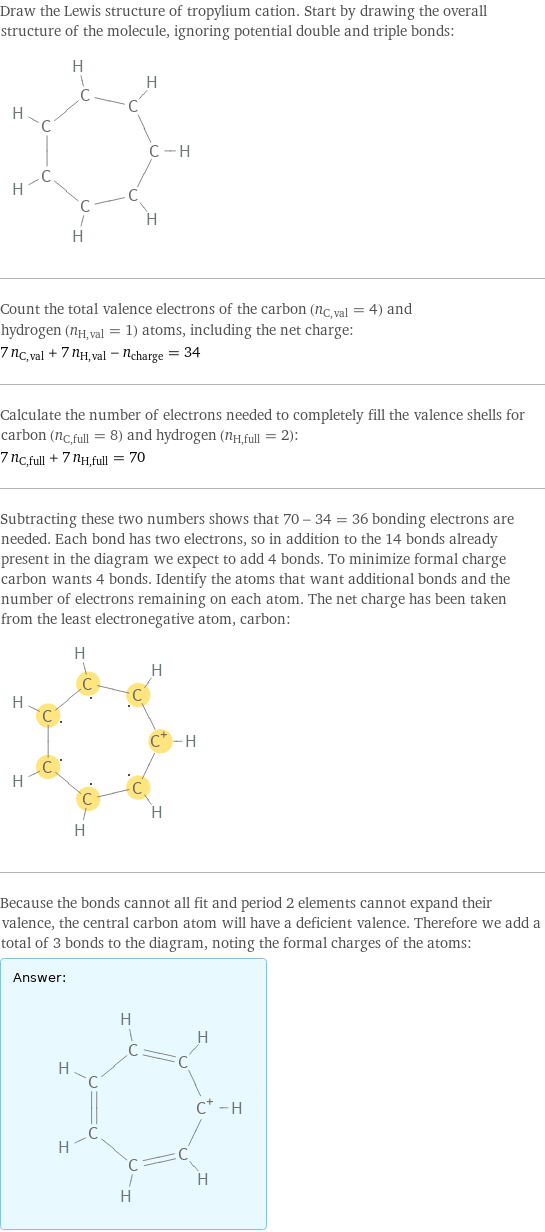
Draw the Lewis structure of tropylium cation. Start by drawing the overall structure of the molecule, ignoring potential double and triple bonds: Count the total valence electrons of the carbon (n_C, val = 4) and hydrogen (n_H, val = 1) atoms, including the net charge: 7 n_C, val + 7 n_H, val - n_charge = 34 Calculate the number of electrons needed to completely fill the valence shells for carbon (n_C, full = 8) and hydrogen (n_H, full = 2): 7 n_C, full + 7 n_H, full = 70 Subtracting these two numbers shows that 70 - 34 = 36 bonding electrons are needed. Each bond has two electrons, so in addition to the 14 bonds already present in the diagram we expect to add 4 bonds. To minimize formal charge carbon wants 4 bonds. Identify the atoms that want additional bonds and the number of electrons remaining on each atom. The net charge has been taken from the least electronegative atom, carbon: Because the bonds cannot all fit and period 2 elements cannot expand their valence, the central carbon atom will have a deficient valence. Therefore we add a total of 3 bonds to the diagram, noting the formal charges of the atoms: Answer: | |
General properties
![formula | ([C_7H_7])^+ net ionic charge | +1 alternate names | tropylium | tropylium(1+)](../image_source/00abe037ac6177222615265c1e913893.png)
formula | ([C_7H_7])^+ net ionic charge | +1 alternate names | tropylium | tropylium(1+)
Other properties

ion class | aromatic | cations | ionic ligands