Input interpretation
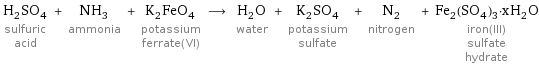
H_2SO_4 sulfuric acid + NH_3 ammonia + K_2FeO_4 potassium ferrate(VI) ⟶ H_2O water + K_2SO_4 potassium sulfate + N_2 nitrogen + Fe_2(SO_4)_3·xH_2O iron(III) sulfate hydrate
Chemical names and formulas
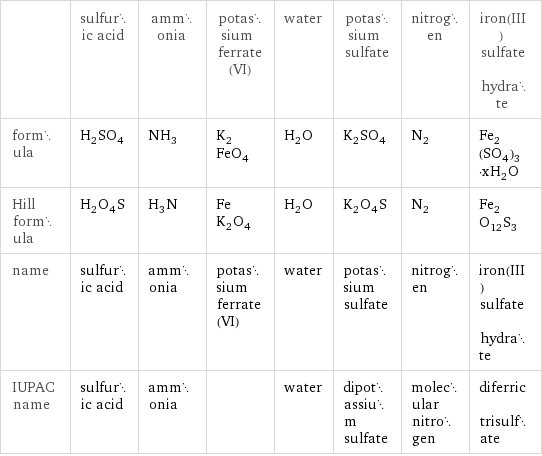
| sulfuric acid | ammonia | potassium ferrate(VI) | water | potassium sulfate | nitrogen | iron(III) sulfate hydrate formula | H_2SO_4 | NH_3 | K_2FeO_4 | H_2O | K_2SO_4 | N_2 | Fe_2(SO_4)_3·xH_2O Hill formula | H_2O_4S | H_3N | FeK_2O_4 | H_2O | K_2O_4S | N_2 | Fe_2O_12S_3 name | sulfuric acid | ammonia | potassium ferrate(VI) | water | potassium sulfate | nitrogen | iron(III) sulfate hydrate IUPAC name | sulfuric acid | ammonia | | water | dipotassium sulfate | molecular nitrogen | diferric trisulfate