Input interpretation
Lewis acids
Members
diborane | boron trifluoride | sulfur trioxide | aluminum fluoride | silicon tetrafluoride | aluminum chloride | iron(III) chloride | titanium tetrachloride | triphenylborane | stannic chloride (total: 10)
Structure diagrams
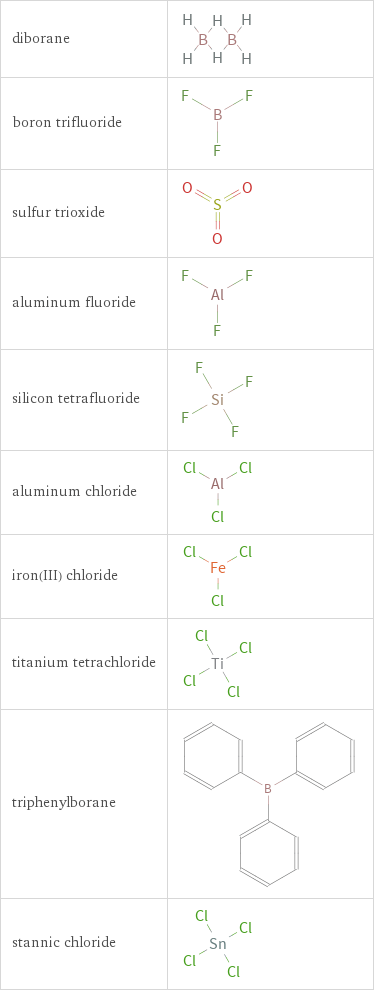
Structure diagrams
Basic properties
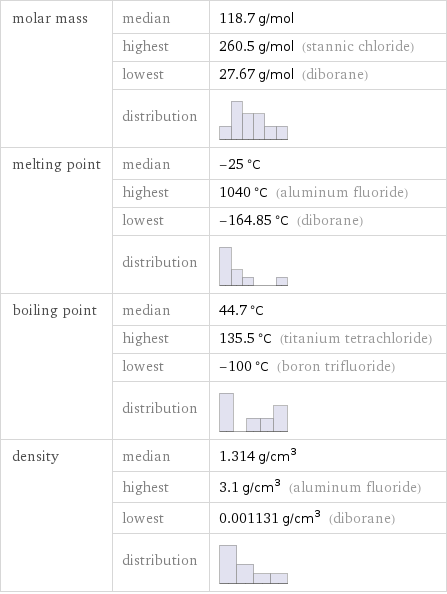
molar mass | median | 118.7 g/mol | highest | 260.5 g/mol (stannic chloride) | lowest | 27.67 g/mol (diborane) | distribution | melting point | median | -25 °C | highest | 1040 °C (aluminum fluoride) | lowest | -164.85 °C (diborane) | distribution | boiling point | median | 44.7 °C | highest | 135.5 °C (titanium tetrachloride) | lowest | -100 °C (boron trifluoride) | distribution | density | median | 1.314 g/cm^3 | highest | 3.1 g/cm^3 (aluminum fluoride) | lowest | 0.001131 g/cm^3 (diborane) | distribution |
Units
