Input interpretation
potassium periodate
Chemical names and formulas
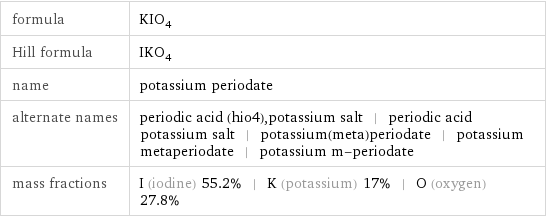
formula | KIO_4 Hill formula | IKO_4 name | potassium periodate alternate names | periodic acid (hio4), potassium salt | periodic acid potassium salt | potassium(meta)periodate | potassium metaperiodate | potassium m-periodate mass fractions | I (iodine) 55.2% | K (potassium) 17% | O (oxygen) 27.8%
Structure diagram
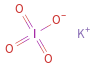
Structure diagram
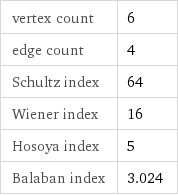
vertex count | 6 edge count | 4 Schultz index | 64 Wiener index | 16 Hosoya index | 5 Balaban index | 3.024
Basic properties
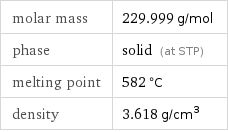
molar mass | 229.999 g/mol phase | solid (at STP) melting point | 582 °C density | 3.618 g/cm^3
Units

Solid properties (at STP)

density | 3.618 g/cm^3
Units

Thermodynamic properties

specific free energy of formation Δ_fG° | solid | -1.571 kJ/g specific heat of formation Δ_fH° | solid | -2.031 kJ/g (at STP)
Units

Chemical identifiers
![CAS number | 7790-21-8 PubChem CID number | 516896 SMILES identifier | [O-]I(=O)(=O)=O.[K+]](../image_source/b5570022b90bf2ccd8d583f61ba14fd4.png)
CAS number | 7790-21-8 PubChem CID number | 516896 SMILES identifier | [O-]I(=O)(=O)=O.[K+]
NFPA label

NFPA label
Ion equivalents

K^+ (potassium cation) | 1 (IO_4)^- (periodate anion) | 1