Input interpretation
diatomic molecules
Members
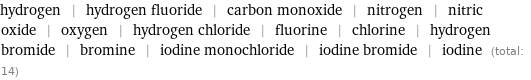
hydrogen | hydrogen fluoride | carbon monoxide | nitrogen | nitric oxide | oxygen | hydrogen chloride | fluorine | chlorine | hydrogen bromide | bromine | iodine monochloride | iodine bromide | iodine (total: 14)
Basic properties
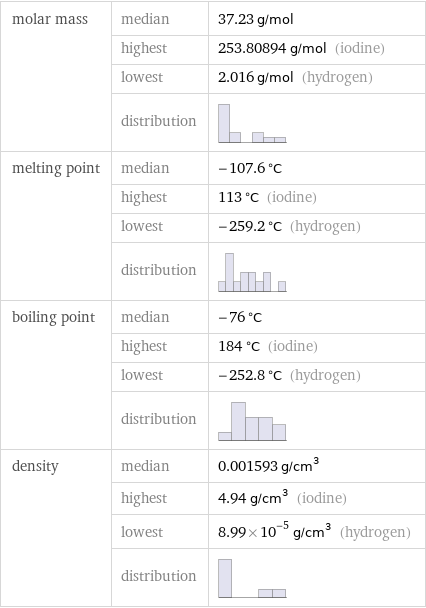
molar mass | median | 37.23 g/mol | highest | 253.80894 g/mol (iodine) | lowest | 2.016 g/mol (hydrogen) | distribution | melting point | median | -107.6 °C | highest | 113 °C (iodine) | lowest | -259.2 °C (hydrogen) | distribution | boiling point | median | -76 °C | highest | 184 °C (iodine) | lowest | -252.8 °C (hydrogen) | distribution | density | median | 0.001593 g/cm^3 | highest | 4.94 g/cm^3 (iodine) | lowest | 8.99×10^-5 g/cm^3 (hydrogen) | distribution |
Units
