Input interpretation
α-berkelium | vanadium(II) oxide
Chemical names and formulas
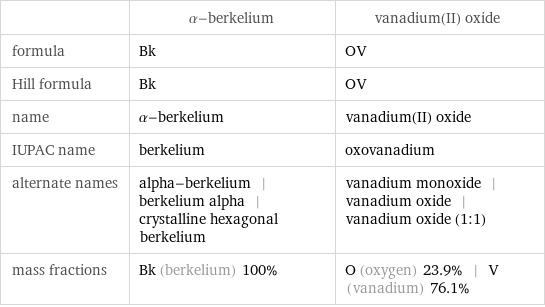
| α-berkelium | vanadium(II) oxide formula | Bk | OV Hill formula | Bk | OV name | α-berkelium | vanadium(II) oxide IUPAC name | berkelium | oxovanadium alternate names | alpha-berkelium | berkelium alpha | crystalline hexagonal berkelium | vanadium monoxide | vanadium oxide | vanadium oxide (1:1) mass fractions | Bk (berkelium) 100% | O (oxygen) 23.9% | V (vanadium) 76.1%
Structure diagrams

Structure diagrams
Basic properties
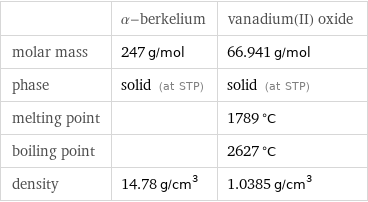
| α-berkelium | vanadium(II) oxide molar mass | 247 g/mol | 66.941 g/mol phase | solid (at STP) | solid (at STP) melting point | | 1789 °C boiling point | | 2627 °C density | 14.78 g/cm^3 | 1.0385 g/cm^3
Units

Thermodynamic properties
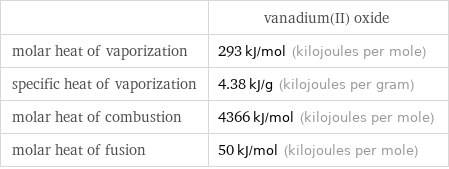
| vanadium(II) oxide molar heat of vaporization | 293 kJ/mol (kilojoules per mole) specific heat of vaporization | 4.38 kJ/g (kilojoules per gram) molar heat of combustion | 4366 kJ/mol (kilojoules per mole) molar heat of fusion | 50 kJ/mol (kilojoules per mole)