Input interpretation
tin(IV) sulfide | praseodymium(III) nitrate, hexahydrate
Chemical names and formulas
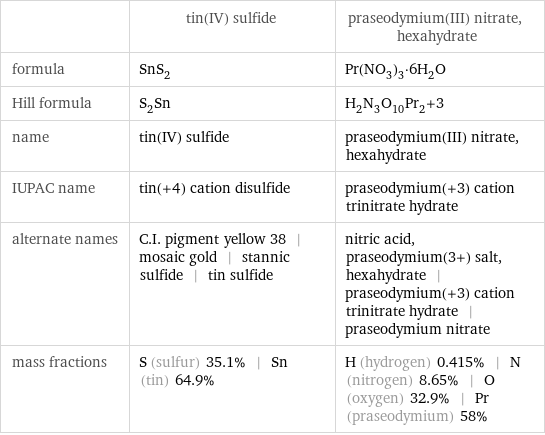
| tin(IV) sulfide | praseodymium(III) nitrate, hexahydrate formula | SnS_2 | Pr(NO_3)_3·6H_2O Hill formula | S_2Sn | H_2N_3O_10Pr_2+3 name | tin(IV) sulfide | praseodymium(III) nitrate, hexahydrate IUPAC name | tin(+4) cation disulfide | praseodymium(+3) cation trinitrate hydrate alternate names | C.I. pigment yellow 38 | mosaic gold | stannic sulfide | tin sulfide | nitric acid, praseodymium(3+) salt, hexahydrate | praseodymium(+3) cation trinitrate hydrate | praseodymium nitrate mass fractions | S (sulfur) 35.1% | Sn (tin) 64.9% | H (hydrogen) 0.415% | N (nitrogen) 8.65% | O (oxygen) 32.9% | Pr (praseodymium) 58%
Structure diagrams
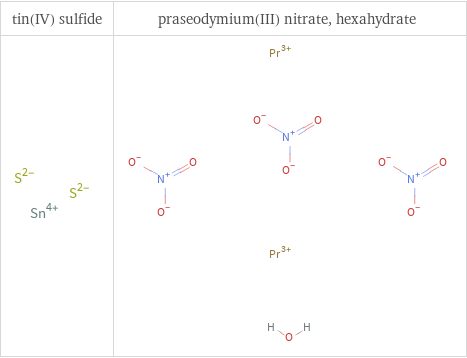
Structure diagrams
Basic properties
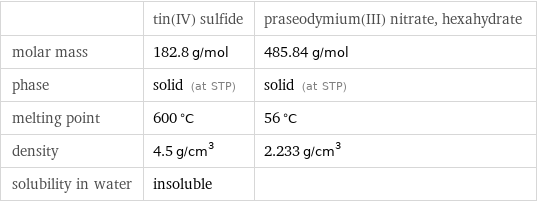
| tin(IV) sulfide | praseodymium(III) nitrate, hexahydrate molar mass | 182.8 g/mol | 485.84 g/mol phase | solid (at STP) | solid (at STP) melting point | 600 °C | 56 °C density | 4.5 g/cm^3 | 2.233 g/cm^3 solubility in water | insoluble |
Units
