Input interpretation
paramagnetic elements | number of valence electrons
Summary
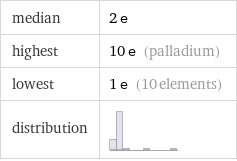
median | 2 e highest | 10 e (palladium) lowest | 1 e (10 elements) distribution |
Units

Number of valence electrons rankings
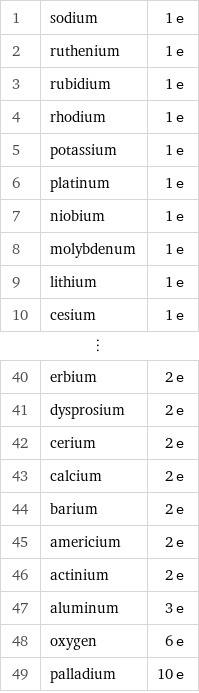
1 | sodium | 1 e 2 | ruthenium | 1 e 3 | rubidium | 1 e 4 | rhodium | 1 e 5 | potassium | 1 e 6 | platinum | 1 e 7 | niobium | 1 e 8 | molybdenum | 1 e 9 | lithium | 1 e 10 | cesium | 1 e ⋮ | | 40 | erbium | 2 e 41 | dysprosium | 2 e 42 | cerium | 2 e 43 | calcium | 2 e 44 | barium | 2 e 45 | americium | 2 e 46 | actinium | 2 e 47 | aluminum | 3 e 48 | oxygen | 6 e 49 | palladium | 10 e
Corresponding quantity
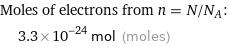
Moles of electrons from n = N/N_A: | 3.3×10^-24 mol (moles)