Input interpretation

vanadium tetrachloride
Chemical names and formulas
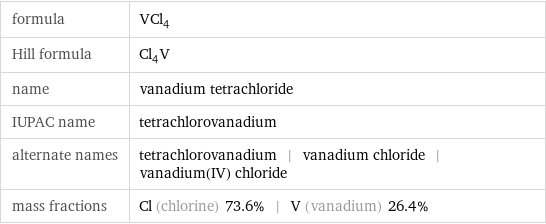
formula | VCl_4 Hill formula | Cl_4V name | vanadium tetrachloride IUPAC name | tetrachlorovanadium alternate names | tetrachlorovanadium | vanadium chloride | vanadium(IV) chloride mass fractions | Cl (chlorine) 73.6% | V (vanadium) 26.4%
Structure diagram
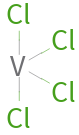
Structure diagram
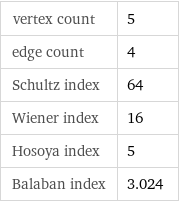
vertex count | 5 edge count | 4 Schultz index | 64 Wiener index | 16 Hosoya index | 5 Balaban index | 3.024
Basic properties
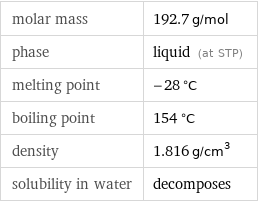
molar mass | 192.7 g/mol phase | liquid (at STP) melting point | -28 °C boiling point | 154 °C density | 1.816 g/cm^3 solubility in water | decomposes
Units

Liquid properties (at STP)

density | 1.816 g/cm^3 vapor pressure | 8 mmHg
Units

Thermodynamic properties
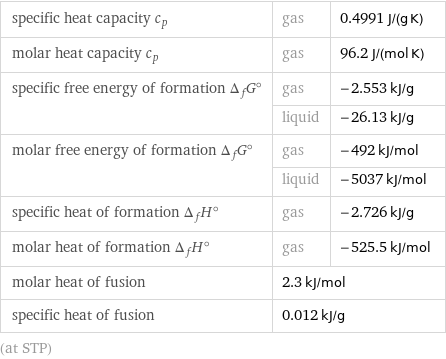
specific heat capacity c_p | gas | 0.4991 J/(g K) molar heat capacity c_p | gas | 96.2 J/(mol K) specific free energy of formation Δ_fG° | gas | -2.553 kJ/g | liquid | -26.13 kJ/g molar free energy of formation Δ_fG° | gas | -492 kJ/mol | liquid | -5037 kJ/mol specific heat of formation Δ_fH° | gas | -2.726 kJ/g molar heat of formation Δ_fH° | gas | -525.5 kJ/mol molar heat of fusion | 2.3 kJ/mol | specific heat of fusion | 0.012 kJ/g | (at STP)
Chemical identifiers
(Cl)Cl InChI identifier | InChI=1/4ClH.V/h4*1H;/q;;;;+4/p-4/f4Cl.V/h4*1h;/q4*-1;m/rCl4V/c1-5(2, 3)4 RTECS number | YW2625000 MDL number | MFCD00061343](../image_source/a4aed54238126875533a3405c58c08fd.png)
CAS number | 7632-51-1 PubChem CID number | 24273 PubChem SID number | 24862677 SMILES identifier | Cl[V](Cl)(Cl)Cl InChI identifier | InChI=1/4ClH.V/h4*1H;/q;;;;+4/p-4/f4Cl.V/h4*1h;/q4*-1;m/rCl4V/c1-5(2, 3)4 RTECS number | YW2625000 MDL number | MFCD00061343
NFPA label

NFPA label
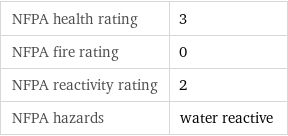
NFPA health rating | 3 NFPA fire rating | 0 NFPA reactivity rating | 2 NFPA hazards | water reactive
Toxicity properties

RTECS classes | mutagen