Input interpretation
lead(II) chromate | yttrium(III) perchlorate
Chemical names and formulas
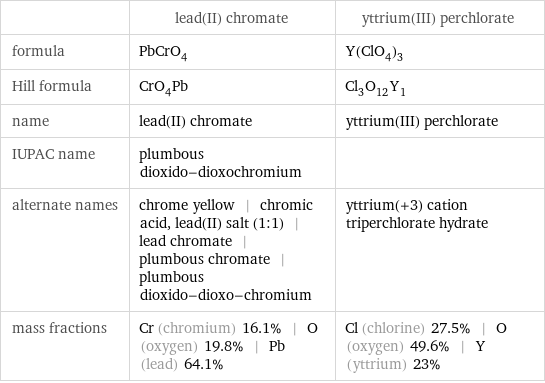
| lead(II) chromate | yttrium(III) perchlorate formula | PbCrO_4 | Y(ClO_4)_3 Hill formula | CrO_4Pb | Cl_3O_12Y_1 name | lead(II) chromate | yttrium(III) perchlorate IUPAC name | plumbous dioxido-dioxochromium | alternate names | chrome yellow | chromic acid, lead(II) salt (1:1) | lead chromate | plumbous chromate | plumbous dioxido-dioxo-chromium | yttrium(+3) cation triperchlorate hydrate mass fractions | Cr (chromium) 16.1% | O (oxygen) 19.8% | Pb (lead) 64.1% | Cl (chlorine) 27.5% | O (oxygen) 49.6% | Y (yttrium) 23%
Structure diagrams
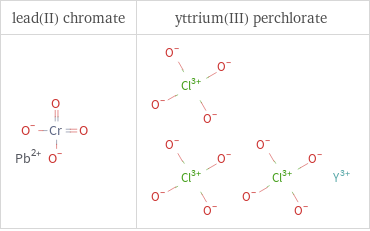
Structure diagrams
Basic properties
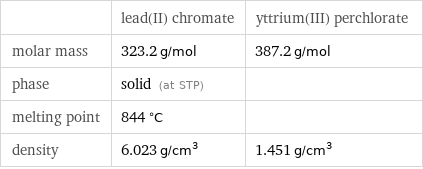
| lead(II) chromate | yttrium(III) perchlorate molar mass | 323.2 g/mol | 387.2 g/mol phase | solid (at STP) | melting point | 844 °C | density | 6.023 g/cm^3 | 1.451 g/cm^3
Units

Solid properties
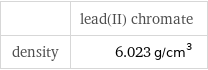
| lead(II) chromate density | 6.023 g/cm^3
Units
