Input interpretation
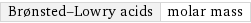
Brønsted-Lowry acids | molar mass
Summary
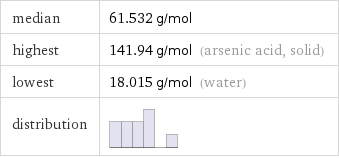
median | 61.532 g/mol highest | 141.94 g/mol (arsenic acid, solid) lowest | 18.015 g/mol (water) distribution |
Units

Distribution plots
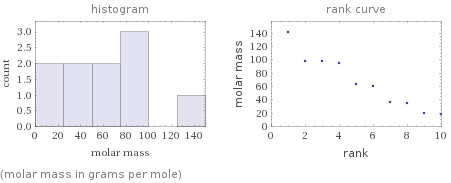
(molar mass in grams per mole)
Molar mass rankings
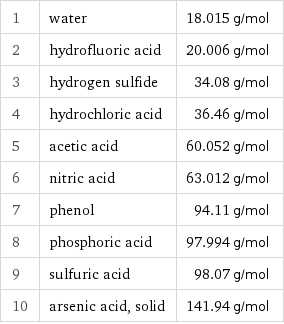
1 | water | 18.015 g/mol 2 | hydrofluoric acid | 20.006 g/mol 3 | hydrogen sulfide | 34.08 g/mol 4 | hydrochloric acid | 36.46 g/mol 5 | acetic acid | 60.052 g/mol 6 | nitric acid | 63.012 g/mol 7 | phenol | 94.11 g/mol 8 | phosphoric acid | 97.994 g/mol 9 | sulfuric acid | 98.07 g/mol 10 | arsenic acid, solid | 141.94 g/mol
Unit conversion for median molar mass 61.532 g/mol
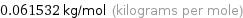
0.061532 kg/mol (kilograms per mole)
Comparisons for median molar mass 61.532 g/mol
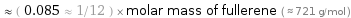
≈ ( 0.085 ≈ 1/12 ) × molar mass of fullerene ( ≈ 721 g/mol )
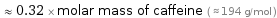
≈ 0.32 × molar mass of caffeine ( ≈ 194 g/mol )
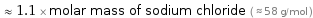
≈ 1.1 × molar mass of sodium chloride ( ≈ 58 g/mol )
Corresponding quantities
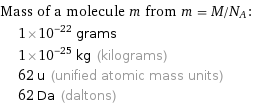
Mass of a molecule m from m = M/N_A: | 1×10^-22 grams | 1×10^-25 kg (kilograms) | 62 u (unified atomic mass units) | 62 Da (daltons)
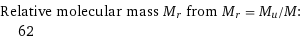
Relative molecular mass M_r from M_r = M_u/M: | 62