Input interpretation

period 6 elements | atomic mass
Summary
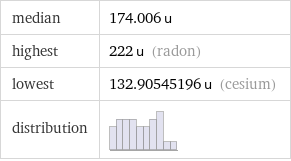
median | 174.006 u highest | 222 u (radon) lowest | 132.90545196 u (cesium) distribution |
Units

Distribution plots
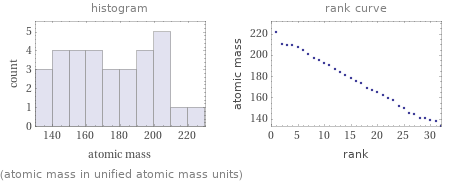
(atomic mass in unified atomic mass units)
Atomic mass rankings
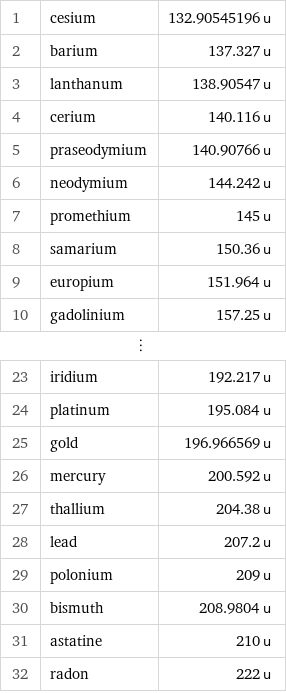
1 | cesium | 132.90545196 u 2 | barium | 137.327 u 3 | lanthanum | 138.90547 u 4 | cerium | 140.116 u 5 | praseodymium | 140.90766 u 6 | neodymium | 144.242 u 7 | promethium | 145 u 8 | samarium | 150.36 u 9 | europium | 151.964 u 10 | gadolinium | 157.25 u ⋮ | | 23 | iridium | 192.217 u 24 | platinum | 195.084 u 25 | gold | 196.966569 u 26 | mercury | 200.592 u 27 | thallium | 204.38 u 28 | lead | 207.2 u 29 | polonium | 209 u 30 | bismuth | 208.9804 u 31 | astatine | 210 u 32 | radon | 222 u
Unit conversions for median atomic mass 174.006 u
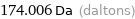
174.006 Da (daltons)
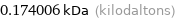
0.174006 kDa (kilodaltons)
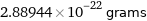
2.88944×10^-22 grams
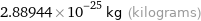
2.88944×10^-25 kg (kilograms)

174.013 chemical atomic mass units (unit officially deprecated)

174.061 physical atomic mass units (unit officially deprecated)
Comparison for median atomic mass 174.006 u
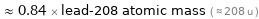
≈ 0.84 × lead-208 atomic mass ( ≈ 208 u )
Corresponding quantities
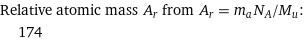
Relative atomic mass A_r from A_r = m_aN_A/M_u: | 174
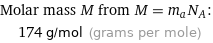
Molar mass M from M = m_aN_A: | 174 g/mol (grams per mole)