Input interpretation
rhodium(III) oxide | lead iodide
Chemical names and formulas
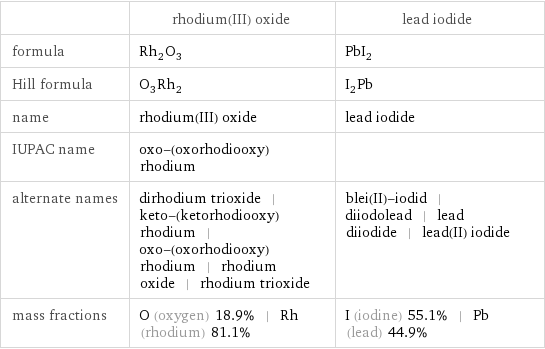
| rhodium(III) oxide | lead iodide formula | Rh_2O_3 | PbI_2 Hill formula | O_3Rh_2 | I_2Pb name | rhodium(III) oxide | lead iodide IUPAC name | oxo-(oxorhodiooxy)rhodium | alternate names | dirhodium trioxide | keto-(ketorhodiooxy)rhodium | oxo-(oxorhodiooxy)rhodium | rhodium oxide | rhodium trioxide | blei(II)-iodid | diiodolead | lead diiodide | lead(II) iodide mass fractions | O (oxygen) 18.9% | Rh (rhodium) 81.1% | I (iodine) 55.1% | Pb (lead) 44.9%
Structure diagrams
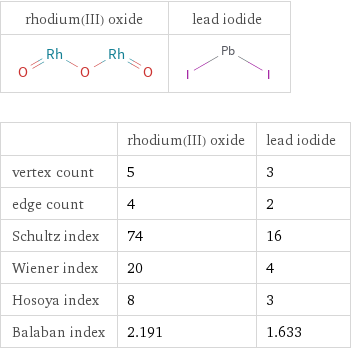
| rhodium(III) oxide | lead iodide vertex count | 5 | 3 edge count | 4 | 2 Schultz index | 74 | 16 Wiener index | 20 | 4 Hosoya index | 8 | 3 Balaban index | 2.191 | 1.633
Basic properties
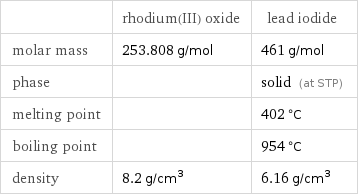
| rhodium(III) oxide | lead iodide molar mass | 253.808 g/mol | 461 g/mol phase | | solid (at STP) melting point | | 402 °C boiling point | | 954 °C density | 8.2 g/cm^3 | 6.16 g/cm^3
Units

Thermodynamic properties
| lead iodide molar heat of vaporization | 104 kJ/mol (kilojoules per mole) specific heat of vaporization | 0.226 kJ/g (kilojoules per gram) molar heat of fusion | 23.4 kJ/mol (kilojoules per mole)