Input interpretation

ionic diatomics
Members
lithium hydride | sodium hydride | boron nitride | lithium chloride | potassium chloride | rubidium fluoride | potassium bromide | rubidium chloride | lithium iodide | cesium iodide (total: 10)
Structure diagrams
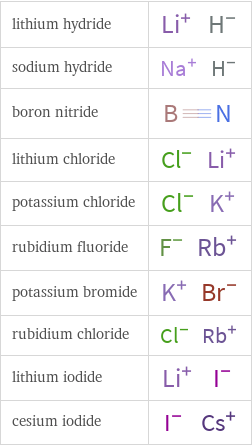
Structure diagrams
Basic properties
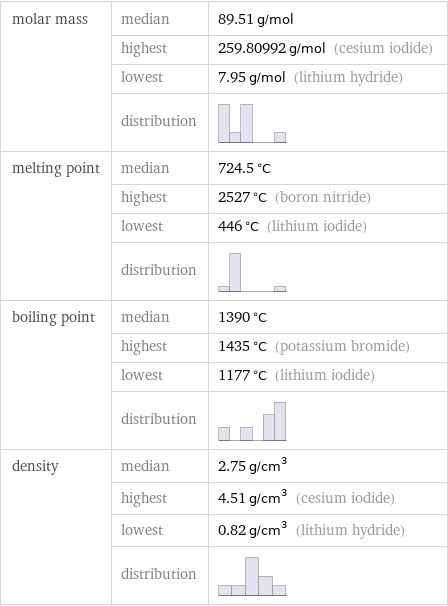
molar mass | median | 89.51 g/mol | highest | 259.80992 g/mol (cesium iodide) | lowest | 7.95 g/mol (lithium hydride) | distribution | melting point | median | 724.5 °C | highest | 2527 °C (boron nitride) | lowest | 446 °C (lithium iodide) | distribution | boiling point | median | 1390 °C | highest | 1435 °C (potassium bromide) | lowest | 1177 °C (lithium iodide) | distribution | density | median | 2.75 g/cm^3 | highest | 4.51 g/cm^3 (cesium iodide) | lowest | 0.82 g/cm^3 (lithium hydride) | distribution |
Units
